Received: Mon 07, Aug 2023
Accepted: Tue 29, Aug 2023
Abstract
RNA-binding motif protein 3 (RBM3) is a cold stress-induced glycine-rich RNA-binding protein that plays a crucial role in regulating global protein synthesis and is involved in cold stress-induced responses, neuroprotection, cytoprotection, antiapoptotic processes, and cell proliferation. RBM3 plays an essential role in tumor progression and metastasis. In this review, we explored the structure, dynamic distribution, neuroprotective functions under mild hypothermia, and cytoprotective functions under adverse stress conditions of RBM3, as well as its regulatory role in tumor progression and metastasis based on findings from recent related research.
Keywords
RBM3, cold stress-induced protein, physiological functions, cancers
1. Introduction
RNA-binding motif protein 3 (RBM3) is an important cold shock protein (CSP) that plays a key role in an organism’s rapid adaptation to environmental stress. The upregulation and reduced global expression of RBM3 are associated with mild hypothermia. RBM3 is a highly conserved RNA-binding protein (RBP) associated with capping, pre-mRNA splicing, 3′ end cleavage and polyadenylation, messenger RNA (mRNA) export, mRNA stability, and mRNA translation [1]. RBM3 has multiple functions, including increasing global protein synthesis during mild hypothermic conditions [2], neuroprotection [3-9], cytoprotection, promotion of cell survival [10-17], cell proliferation [7-11, 18-21], and modulation of cancer. In addition to promoting cell proliferation and survival in response to stress-induced inflammation [22, 23] and acting as an RNA chaperone of RNA stability [18, 24-27] during carcinogenesis, RBM3 is a proto‐oncogene [18]. Numerous studies have reported the upregulation of RBM3 expression in various cancers. The role of RBM3 as a promising diagnostic marker and a potential treatment-predictive target for different cancers has been recognized. Other functions of RBM3 include its role in the circadian rhythm [28] and spermatogenesis [1]. This review aimed to consider the protective roles of RBM3 and their underlying mechanism in different disease conditions, including cancers by appraising recent literature in these fields.
2. RBM3 Structure and Function
RBM3 is an important CSP that has been identified through complementary DNA (cDNA) selection in plants, yeast, and mammals [29]. It is a highly conserved RBP and its transcription is upregulated in response to mild hypothermia [30]. Located between OATL1 and GATA1/TFE3 in the subband Xp11.23, RBM3 forms alternatively spliced transcripts in various human tissues. The longest open reading frame encodes 157 amino acid proteins with a predicted molecular weight of 17 kDa [29]. RBM3 has two important domains: N-terminal RNA recognition motif (RNA recognition motif (RRM) domain; 85 amino acids) and C-terminal arginine glycine-rich (RGG domain; 72 amino acids) domains. The RRM domain adopts a βαββαβ topology [31, 32] containing two ribonucleoprotein domains (RNPs), RNP1 and RNP2, which are located at the N-terminal protein end. The consensus sequences of RNP1 and RNP2 are (K/R) G(F/Y) (G/A) FVX(FY) and (L/I) (F/Y) (V/I) (G/K) (G/N)L, respectively [30].
At the subcellular level, RBM3 is mainly located in the nucleus [30]; however, it can shuttle between the nucleus and cytoplasm in response to adverse conditions [18]. RBM3 is expressed in multiple brain regions, with the cerebellum and olfactory bulb having the highest expression of RBM3 [33]. In mammals, RBM3 levels peak during the early postnatal period and thereafter decrease to very low levels during youth and adulthood in most regions of the brain, except for areas where proliferation remains active, such as the subventricular zone and hippocampal subgranular zones (SGZ) [30]. In the testes of adult mice, RBM3 mRNA and protein were detected in Sertoli cells but not in the germ cells of seminiferous tubules at all stages [34]. Mechanistically, RBM3 binds to 60S ribosomal subunits, alters microRNA levels, dephosphorylates eukaryotic initiation factor 2 alpha (eIF2α), promotes the phosphorylation of eukaryotic initiation factor, and enhances global protein synthesis [35].
2.1. Multiple Physiological Functions of RBM3 in Response to Cold Stress
Global protein synthesis and cellular metabolism are generally suppressed in response to mild hypothermia. However, the expression of RBM3, a cold stress-induced protein, was enhanced [2]. Induced mild hypothermia, an effective brain protection method, has been used to treat patients with severe hypoxic-ischemic encephalopathy and brain injury, and those who receive cardiac surgery and cardiopulmonary resuscitation.
However, some mild hypothermia-related complications, including myokymia, immune dysfunction, respiratory tract infection, bedsores, arrhythmia, unstable circulation, increased rebound intracranial pressure, coagulation dysfunction, and electrolyte disorders may occur, which strongly limit the clinical application of induced mild hypothermia.
Numerous studies have reported the neuroprotective effect of increasing RBM3 expression through therapeutic hypothermia. Thus, the selective upregulation of RBM3 under normothermic conditions holds extraordinary therapeutic potential. A recent study confirmed that hypothermia increases RBM3 and reticulon 3 expression, improving cerebellar-dependent learning following hypoxic ischemia incidence in neonatal rats [36]. One study suggested the possibility of manipulating RBM3 expression by modulating the alternative splicing of E3a using antisense oligonucleotides, identifying E3a as a potential therapeutic target for neuroprotection due to increased RBM3 expression during normothermic conditions [37].
Moreover, RBM3 can potentially mediate the regulation of body temperature during autophagy deficiency [38]. A previous study indicated that in a mouse model of traumatic brain injury (TBI), hypothermic pretreatment (HT) can reverse deficits in long-term potentiation (LTP) and cognition, loss of spines, and abnormal tau phosphorylation at several sites [3], and limiting RBM3 expression using RBM3 short hairpin RNA eliminated the neuroprotective effect of HT in TBI mouse models. In contrast, HT-like neuroprotection against TBI-induced chronic brain injury can be mimicked by overexpressing RBM3 using an AAV-RBM3 plasmid [3]. RBM3 increases cell survival in brain injury models, mainly by inhibiting cellular apoptosis [4, 5] and enhancing the activation of extracellular signal-related kinase signaling pathways [6]. A previous study confirmed that RBM3 in the embryo regulates the proliferation and differentiation of neural stem cells (NSCs) to promote fetal cerebral cortex development during maternal cold stress. The mechanism underlying these effects may be the orchestration of neurogenesis by the binding of RBM3 to the Yap1-3′UTR, which regulate its mRNA stability and promote YAP1 expression [26]. Similarly, a study showed that in a mouse model of alzheimer’s and prion diseases, RBM3 mediated structural plasticity and the protective effects of hypothermia-induced neuroprotection, thus delaying neurodegenerative disease development [8]. Moreover, hypothermia induces RBM3 through TrkB-PLCγ1-CREB-RBM3 signaling for structural plasticity mediation, which is reversed in RBM3-null neurons [39].
Experimental and clinical data indicate that targeted temperature management (TTM) can significantly induce RBM3 mRNA expression in blood samples of patients with post-cardiac arrest. However, upregulated RBM3 expression was not observed in response to TTM. One possible hypothesis is that RBM3 is mainly located intracellularly and is not actively released into the circulatory system, and any changes in its expression can be measured in the serum [2]. Therefore, the mechanism underlying the active secretion of RBM3 requires further investigation, and RBM3 expression should be measured in full blood samples and not in serum samples only.
Numerous studies have shown that hypothermia-induced upregulated expression of RBM3 induced by hypothermia plays an active role in cell protection [4-6, 8, 9, 32, 33]. In response to cold stress, the liver increases the relative expression of RBM3 mRNA, promoting cell survival [12]. In mice, O-GlcNAcylation of p65 contributes to RBM3 upregulation during mild hypothermia and promotes protein kinase B (AKT) phosphorylation to enhance glucose metabolism and inhibit apoptosis in the skeletal muscles of mice [17]. A recent study demonstrated that RBM3 might exert a critical influence on adaptive post-transcriptional regulatory processes linked to the maintenance of muscle mass and function [40].
2.2. RBM3 Bioactivities Beyond Hypothermia
Several studies have suggested that RBM3 exerts cytoprotective effects in various stress conditions, inhibits apoptosis, and promotes cell survival. RBM3 alleviates apoptosis and oxidative stress in acute lung injury [41]. Moreover, it protects human SH-SY5Y neuroblastoma cells from nitric oxide-induced apoptosis by modulating p38 signaling and miR-143 expression [13]. RBM3 protects neuroblastoma cells against UV irradiation-induced apoptosis by inhibiting the activation of the proapoptotic signaling pathways, p38 and JNK, and downregulating the expression of the pro-apoptotic proteins, bax and bad [15]. Overexpression of RBM3 inhibits the induction of necrosis, apoptosis, and apoptosis-associated caspases, enhancing the survival of skeletal muscle myoblasts [14].
In HI brain injury, RBM3 promotes neural stem/progenitor cell (NSPC) proliferation in the SGZ via the IMP2-IGF2 signaling pathway and is involved in neuroprotection and neurogenesis [7]. RBM3 reverses apoptosis induced by oxygen-glucose deprivation/reoxygenation (OGD/R)-related injury by binding to GTPase-activating protein-binding protein 1 (G3BP1) and promoting stress granule (SG) generation in the PC12 cells and rat primary cortical neurons [16]. Recent findings support the protective effects of RBM3 against I/R-induced myocardial apoptosis through the Raptor/Beclin1 pathway, which regulates autophagy [42]. Additionally, RBM3 inhibits PERK phosphorylation in an NF90-dependent manner and modulates the canonical PERK-eIF2a-CHOP endoplasmic reticulum stress pathway to exert cellular protective effects [10]. Overexpression of RBM3 prevents the apoptosis of fibroblast and human embryonic kidney (HEK293) cells during serum starvation, possibly by restoring translation efficacy through 14C serine and 3H phenylalanine incorporation [11].
Meanwhile, Xie et al. found that the 11S proteasome activator, REGγ, promotes aortic dissection (AD) by inhibiting the RBM3-SRF (serum response factor) pathway as RBM3 plays a role in increasing SRF mRNA stability and expression [43]. There is evidence that RBM3 is downregulated in febrile illness and contributes to the increased expression of temperature-sensitive microRNAs involved in the fever response, such as miR-142-5p and miR-143. In turn, miR-1425p and miR-143 suppress the expression of endogenous pyrogens, including IL-6, IL6ST, TLR2, PGE2, and TNF, to complete a negative feedback mechanism that plays an indispensable role in preventing pathological hyperthermia [44]. Another study showed that RBM3 suppresses lung innate lymphoid cell activation and inflammation, which may be partially mediated by the cysteinyl leukotriene 1 receptor [45].
Moreover, another study suggested that RBM3 promotes NSC proliferation in moderately hypoxic conditions by promoting G1 to S phase transition [19]. Other studies indicated that RBM3 plays an indispensable role in the proliferation of fibroblasts and HEK293 [11], breast cancer [20], SW480 colon cancer [18], HCT116 colon adenocarcinoma [46], and hepatocellular carcinoma [47]. RBM3 has morphoregulatory functions relevant to its role in cell protection via RhoA-ROCK-CRMP2 signaling [48]. Considering its parallel influence on cell protection [13-17] and migration [21, 48], RBM3 can be a critical factor in regenerative responses, such as wound healing.
3. Paradoxical Roles of RBM3 in Cancer
Cancer-related morbidity and mortality are increasing worldwide. Cancer is likely to be the leading cause of death and a major barrier to increased life expectancy in every country in the 21st century [49]. Several immunohistochemical studies have demonstrated that RBM3 has high oncogenic potential owing to its increased expression in various human tumors. Recently, RBM3 has been identified as a prognostic biomarker for several types of human cancers. Dysregulated expression of oncogenes and tumor suppressors is the main cause of tumorigenesis, which is a dynamic process involving multifactorial, multistage, and multigene processes. Cancer is a complex polygenic disease, and its occurrence and development are closely related to various stress conditions, such as chronic inflammation, DNA damage, and hypoxia. RBM3 is a stress-induced protein that responds to various stressors and plays different roles in various human cancers.
However, the role of RBM3 in cancer can be dichotomous depending on the cellular context, as it can either enhance or repress tumorigenesis. Increased expression of RBM3 is associated with favorable clinicopathological parameters and prognosis in some cancers, including epithelial ovarian cancer (EOC), malignant melanoma, non-small cell lung cancer (NSCLC), non-seminomatous germ cell tumors (NSGCT), upper gastrointestinal cancers, muscle-invasive bladder cancer treated with neoadjuvant cisplatin-based chemotherapy (NAC) [50-53], and poor prognosis in other cancers, such as nasopharyngeal carcinoma, urothelial bladder cancer (T1 stage), and hepatocellular carcinoma (HCC) [21, 47, 54, 55]. Paradoxically, the expression level and clinical behavior of RBM3 in several cancers, including colorectal (CRC), prostate (PCa), breast, and pancreatic cancers, are still conflicting [18, 20, 24, 56-61]. RBM3 is involved in multiple central processes in cancer biology, including apoptosis, proliferation, and angiogenesis.
3.1. CRC
CRC arises from the loss of intestinal epithelial homeostasis and hyperproliferation of the crypt epithelium [46]. In the HCT 116 and DLD-1 cell lines, doxycycline-induced RBM3 overexpression causes stemness in CRC through a mechanism involving the suppression of GSK3β activity, thereby enhancing β-catenin signaling [46]. Sureban et al. [18] confirmed that RBM3 was significantly upregulated in colorectal tumors in a stage-dependent manner and demonstrated RBM3 as a proto-oncogene when RBM3 overexpression induces non-transformed cells to grow in an anchorage-independent manner [18], which is consistent with the conclusions of Lleonart et al. [62]. In addition, when RBM3 expression is downregulated, tumor growth is greatly suppressed by the decreased expression of the angiogenesis-inducing factors, VEGF and IL-8 [18]. Paradoxically, one study suggested that the downregulated expression of RBM3 in CRC is a predictor of poor prognosis, which is related to right-sided tumor localization [56].
However, the reason for the discrepancy remains unclear. It can be assumed that RBM3 specificity for target genes largely depends on the cancer cell type and molecular background [24, 56, 63]. Further studies are required to determine the role of RBM3 in CRC. Stress response pathways play important roles in cancers caused by the loss of intestinal epithelial homeostasis. An adaptive response can protect the host from various stressors. As a stress response protein, RBM3 is induced in response to various stresses and acts as a chaperone. However, improper stress responses, such as excessive and decreased stress responses, can induce pathogenic conditions, such as inflammation and carcinogenesis [64]. One study found that RBM3 promotes colorectal tumorigenesis in the colonic tissue samples of patients with refractory inflammatory bowel disease and RBM3-deficient (RBM3-/-) mice by inhibiting apoptosis and increasing R-spondin expression in the gut [23]. Refractory and long-term inflammation may occur in the human colonic mucosa, and several studies support the idea that hypoxia contributes to a more malignant tumor phenotype [65-67].
3.2. PCa
PCa is the second most frequently occurring cancer, the fifth leading cause of cancer-related deaths among men in 2020 [49], and a major health issue for men in China. Recently, RBM3 role in tumor development and progression has attracted the attention of researchers. Zeng et al. [24] found that RBM3 expression was significantly increased in cancer lesions but not in adjacent normal glands and suggested that the overexpression of RBM3 in PCa cells due to the inhibition of alternative splicing of exon v8-v10 of CD44 greatly attenuated prostatic stem cell-like features, including proliferation in soft agar or growth under the skin of nude mice [24, 68]. The molecular mechanisms underlying the effects of RBM3 on PCa development were determined by performing high-throughput RNA sequencing (RNA-seq) on RBM3-overexpressing and RBM3-knockdown PCa cells. The gene ontology biological processes analysis showed that the “lipid catabolic process” and the “phospholipid metabolic process” were tightly associated with RBM3-regulation, and the expression of PLAZGZA (secretory PLA2G2A, the key arachidonic acid metabolizing enzyme, belongs to the PLA2 family and it is involved in multiple pathophysiological processes [69], including lipid metabolism, phospholipid metabolism and inflammation, and insulin regulation [70]) is increased in RBM3-knockdown PCa cells.
Given that low RBM3 expression is associated with poor prognosis or aggressive progression in a number of cancer types, including PCa, increased PLA2G2A expression after RBM3 removal may, to an extent, promote tumor progression and, therefore, PLA2G2A may be a downstream effector of RBM3 in cancer [68]. PLA2 family plays important roles in RBM3 regulation during PCa development. Further studies on the regulation of RBM3 by several metabolic pathways, especially those involving the PLA2 family, should be conducted. Moreover, when PCa metastasizes to the bone, overexpressed RBM3 in PCa cells promotes N6-methyladenosine (m6A) methylation of catenin beta 1 (CTNNB1), reducing its RNA stability, decreasing the expression of β-catenin, and suppressing the stemness remodeling of PCa induced by the osteogenic microenvironment [25].
Nonetheless, the molecular mechanism underlying RBM3 regulation in PCa cells remains unclear, and the role of RBM3 in PCa remains controversial. RBM3 has different prognostic effects in patients with PCa. On the one hand, a study on operated PCa showed that high RBM3 expression in poorly differentiated and more invasive PCa, which is tightly linked to ERG activation and phosphatase and tensin homolog deletion, could be used as an independent prognostic marker to predict the risk of biochemical recurrence and disease progression [57]. On the other hand, a clinical study based on immunohistochemistry performed on PCa tissue microarrays showed that the upregulation of RBM3 expression, particularly in the nucleus, was associated with a significantly prolonged time to biochemical recurrence and clinical progression, supporting that RBM3 is a biomarker of good prognosis [58]. In contrast, downregulating RBM3 expression increases the chemotherapeutic susceptibility of PCa cell lines [59]. However, the mechanisms underlying these functions remain unclear. Understanding the effect of RBM3 on PCa cells might be challenging owing to the reported inconsistencies; therefore, further studies are required.
3.3. Breast Cancer
Among women, breast cancer accounts for one in four cancer cases and one in six cancer-related deaths, ranking first in incidence in most countries (159 of 185 countries) [49]. A clinical study’s findings support the role of RBM3 as an important prognostic factor for breast cancer and showed that increased nuclear expression of RBM3 in breast cancer was associated with improved clinical outcomes, such as low-grade, small tumors, estrogen receptor (ER) positivity, and Ki-67 negativity [60]. Increased nuclear expression of RBM3 is associated with improved overall and recurrence-free survival in patients with breast cancer, and RBM3 expression seems to be an independent predictor of disease outcomes in ER-positive breast cancer [60]. However, a study contradicted these findings, showing that even though two studies suggested that the expression of RBM3 was increased in breast cancer tissues more than in normal tissues [20, 60], the role of RBM3 in female breast cancer is paradoxical. Chen et al. [20] found that lymph node metastasis and a high tumor grade in patients with breast cancer were closely related to increased RBM3 expression.
A high level of RBM3 was associated with worse postoperative relapse-free survival (RFS) and overall survival (OS) rates, whereas the knockdown of RBM3 significantly reversed the proliferation and metastasis of breast cancer cell lines. Moreover, the data obtained by Chen et al. indicated that RBM3 upregulates actin-related protein 2/3 complex subunit 2 (ARPC2) by binding to the 3'UTR, contributing to breast cancer progression. ARPC2 is an important oncogene in human gastric cancer cell lines, which can promote the proliferation and invasion of the human gastric cancer cell line MKN-28 [71]. Therefore, it is important to consider the role of the RBM3-ARPC2 pathway in female breast cancer to aid the discovery of novel potential therapy for breast cancer. According to a recent report, the X-chromosome on RBM genes, including RBM3, may constitute a novel family of apoptosis modulators. RBM3 expression was previously shown to be positively correlated with the pro-apoptotic Bax gene in breast cancer, and it appears that RBM3 plays an unknown role in the regulation of apoptosis in breast cancer. Further studies are required to better understand the molecular mechanisms underlying X-chromosome RBM genes in breast cancer [72].
3.4 Ovarian Cancer
Ovarian cancer is the second most common cause of gynecological cancer-related deaths (after cervical uterine cancer) in women worldwide. Epithelial ovarian cancer (EOC) accounts for > 95% of all ovarian malignancies and is generally detected at an advanced stage [73, 74]. Thus, there is an urgent need to develop effective screening options for early detection of epithelial ovarian cancer. A study of two independent patient cohorts showed that increased RBM3 expression was associated with favorable prognosis in EOC and increased sensitivity to cisplatin in ovarian cancer cells [50]. Moreover, their data confirmed that high RBM3 mRNA expression is an independent predictor of significantly improved RFS and OS [50]. Based on this discovery, in-depth studies were performed to further elucidate the functional mechanisms underlying the role of RBM3 in cisplatin-mediated cell death and found that the main effects of RBM3 on cisplatin sensitivity might be reflected in cell cycle distribution rather than apoptosis [50]. Upregulation of RBM3 expression restrains the checkpoint response by inhibiting the levels of DNA damage checkpoint kinases (CHK1 and CHK2) and minichromosome maintenance protein 3 (MCM3) to promote DNA integrity in EOC, as a deficient DNA repair system can inhibit cancer invasion and metastasis [63].
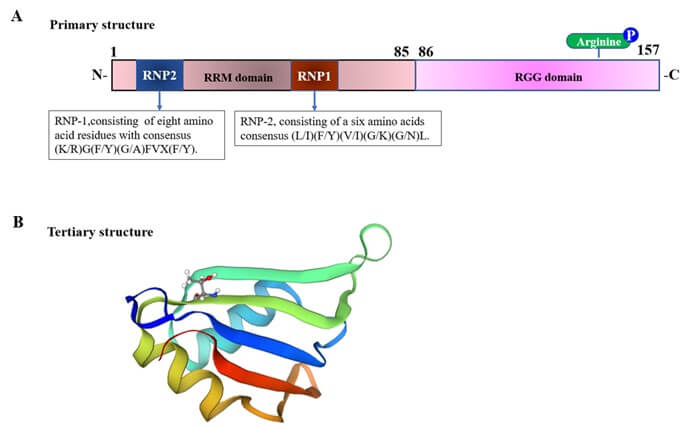
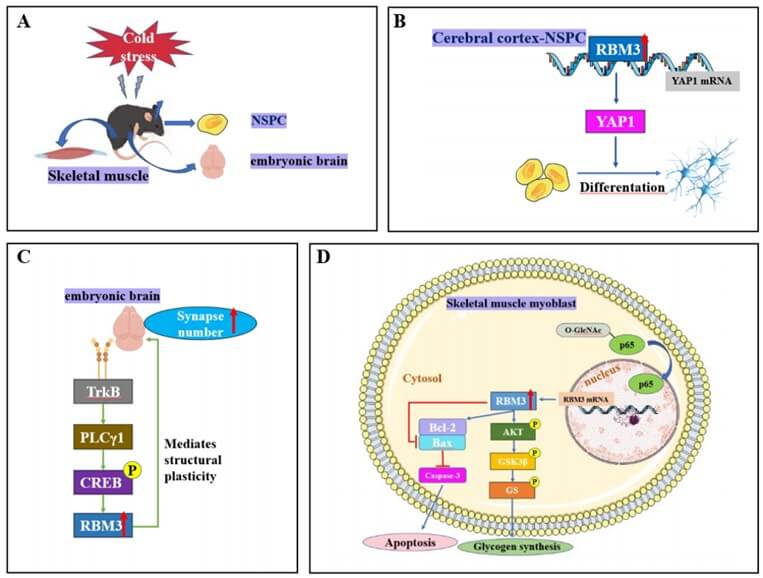
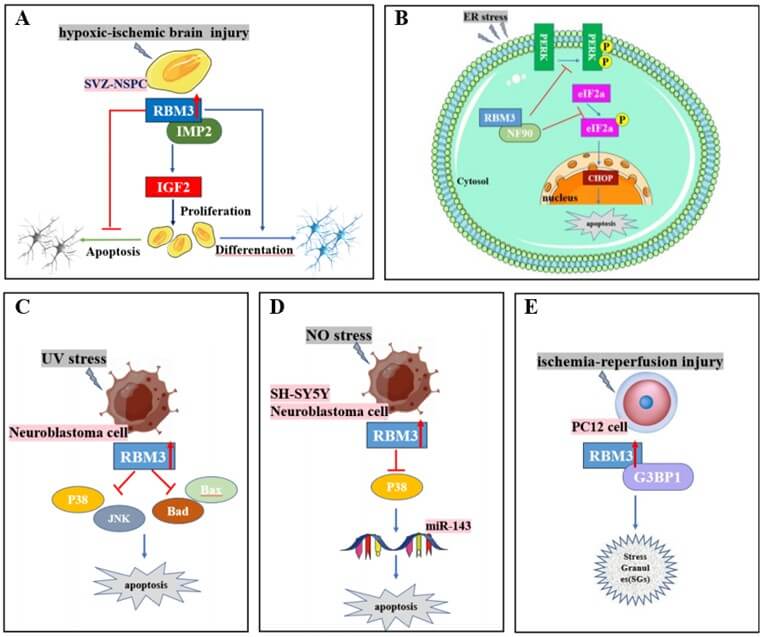
TABLE 1: Prognosis of
RNA‐binding
motif protein 3 (RBM3) expression changes in various types of cancer.
|
Tumor type |
RBM3 expression |
Progosis |
clinical outcome |
Mechanisms
|
References |
|
Colorectal Cancer(CRC) |
Upregulated |
Poor |
Not mentioned |
①GSK3β/β-catenin ②promote VEGF/IL-8
expression |
[46] [18] |
|
Right-sided CRC |
Downregulated |
Poor |
Not mentioned |
Not mentioned |
[56] |
|
Colitis-associated Cancer |
Upregulated
|
Poor
|
Not mentioned |
①Inhibiting
apoptosis and increasing R-spondin ②IBD
refeactory/inflammation/hypoxia |
[23]
[65-67] |
|
Prostate Cancer (PCa) |
①Upregulated
|
Good
|
Not
mentioned |
①Inhibiting
alternative splicing of exon V8-V10 of CD44 to attenuates the prostaftic stem
cell-like features ②RBM3/PLAZG2A ③ERG
activation and PTEN delection ④promotes
N6-methyladenosine(m6A) methylation of catenin beta 1 (CTNNB1) to reduce its
RNA stability and decrease the expression of β-catenin |
[24]
[68] [57]
[25] |
|
②Upregulated |
Good |
prolonged time to biochemical recurrence and
clinical progression |
Not mentioned |
[58] |
|
|
③Downregulated |
Good |
increase chemotherapeutic susceptibility |
Not mentioned |
[59] |
|
|
Breast Cancer(BC) |
①Upregulated(nuclear) |
Good |
①OS/RFS↑②Small
tumor/ER(+)/Ki-67(-) |
Not mentioned |
[60] |
|
②Upregulated |
Poor |
①OS/RFS↓②Lymph node metastasis and
high tumor grade |
RBM3/ARPC2(Binding the 3’UTR of ARPC2 contribute
to BC progression) |
[20] |
|
|
③Unclear |
Unclear |
Unclear |
RBM3 expression is correlation of the
pro-apoptosis Bax gene |
[72] |
|
|
Epithelial Ovarian Cancer(EOC)
|
Upregulated
|
Good |
①OS/RFS↑②Increase cisplatin
sensitivity
|
①Reflect
in cell cycle distribution ②inhibiting
CHK1、CHK2、MCM3,promote DNA integrity |
[50] [63] |
|
Nasopharyngeal carcinoma |
Upregulated |
Poor |
Significantly drives radioresistance |
Inhibiting DNA damage and reduces the rate of
apoptosis via PI3K/AKT/Bcl-2 signaling pathway |
[54] |
|
Urothelial bladder cancer(UBC) |
Downregulated (UBC T1) |
Poor |
Not mentioned |
Not mentioned |
[55] |
|
Muscle invasive bladder cancer(MIBC) |
①Upregulated |
Poor |
Not mentioned |
Not mentioned |
[53] |
|
②Upregulated(received
NAC treatment) |
Good |
①RFS↑②Enhance the sensitivity
to cisplatin and gemcitabine |
Facilitating G1/S-phase translation and initiation
of DNA replication |
[53] |
|
|
Nonseminomatous grem cell tumors(NSGCT) |
Upregulated |
Good |
Enhance the cisplatin sensitivity |
Not mentioned |
[52] |
|
Upper gastrointestinal metastases
|
Upregulated
|
Unclear
|
Promote proliferation
|
Not mentioned |
[77] |
|
Hepatocellular carcinoma(HCC)
|
Upregulated
|
Poor
|
OS/RFS↓
|
①Promoting
HCC cells proliferation in a SCD-CirRNA2/ERK pathway ②RBM3/STAT3/EMT
axis |
[47]
[21] |
|
Pancreatic cancer |
Upregulated |
①Poor |
Enhance migration and invasion |
Not mentioned |
[61] |
|
②Good |
Increase the sensitivity to adjuvant
chemotherapy, in particular gemcitabine |
||||
|
Non-small cell lung cancer(NSCLC) |
Downregulated |
Poor |
Aggressive tumor features |
Not mentioned |
[51] |
|
Upper gastrointestinal cancer |
Downregulated |
Poor |
Promote proliferation |
Not mentioned |
[77] |
|
Malignant melanoma |
Downregulated |
Poor |
OS↓ |
Not mentioned |
[78] |
3.5. Bladder Cancer
Bladder cancer is the 10th most commonly diagnosed cancer worldwide and is more common in men than in women [49]. Urothelial bladder cancer (UBC) is often diagnosed as a non-muscle invasive tumor at the Ta or T1 stages [75]. A validated study found that poor prognosis in patients with UBC at stage T1 was associated with low RBM3 expression, which was considered an independent poor prognostic factor [55]. There is evidence that RBM3 could be a useful biomarker for stratifying patients with non-muscle invasive disease for more aggressive first-line treatments, such as early cystectomy [55]. NAC followed by radical cystectomy is the recommended treatment for muscle-invasive bladder cancer (MIBC) [76]. A cohort study of patients diagnosed with MIBC [53] reported an inferior outcome with an increased risk of MIBC recurrence in patients with high tumor-specific RBM3 expression in transurethral resection of the bladder specimens.
Nevertheless, patients with high RBM3 expression who received NAC had significantly prolonged recurrence-free survival compared to untreated patients [53]. In addition, patients with low RBM3 expression showed a trend toward a higher frequency of pathological downstaging after NAC treatment [53]. High RBM3 expression enhances sensitivity to cisplatin and gemcitabine, which may be associated with RBM3's role in facilitating cell cycle progression, particularly the G1/S-phase transition and initiation of DNA replication [53], which is consistent with the findings on ovarian cancer [63].
3.6. Other Cancers of the Digestive System
Digestive system cancer is an important type of cancer worldwide. A recent study on upper gastrointestinal cancers [77] found that high expression of RBM3 in esophageal and gastric adenocarcinomas was related to intestinal metaplasia-associated tumors, such as benign squamous epithelium and intestinal metaplasia (barrett’s esophagus or gastric intestinal metaplasia), in which RBM3 expression was proven to be an independent prognostic and treatment-predictive biomarker. Low RBM3 expression is an independent factor for reduced overall and recurrence-free survival in patients with upper gastrointestinal tract adenocarcinoma. Compared to primary cancer, there was a significant positive association between higher RBM3 expression and metastases [77]. Conversely, in hepatocellular carcinoma (HCC), RBM3 is significantly upregulated and correlated with poor clinical prognosis, including short recurrence-free survival and poor OS [21, 47].
Regarding the molecular mechanism underlying hepatocarcinogenesis, according to a previous study [47], RBM3 promotes HCC cell proliferation in an SCD-circRNA 2 (a circRNA derived from the 3′UTR of the stearoyl-CoA desaturase (SCD) gene)-dependent manner. Moreover, RBM3 might bind to the SCD mRNA 3′UTR, increasing SCD-circRNA 2 expression depending on the flanking sequence of the backsplicing site, and promoting HCC cell proliferation through the SCD-circRNA 2/ERK pathway. Moreover, Zhang et al. [21] showed that RBM3 promotes EMT-induced tumor migration and invasion and that RBM3 binds directly to the squamous cell carcinoma antigen recognized by T cell 3 (STAT3) mRNA to enhance its stability by activating the RBM3/STAT3/EMT axis, which promotes HCC progression. Moreover, they found that microRNA-383 inhibits RBM3 expression by binding to the 3ʹUTR of RBM3 mRNA. Based on their findings, microRNA-383 was considered an upstream target of RBM3, which may be a new effective therapeutic target for HCC metastasis. Another study [61] revealed a two-faceted role of RBM3 in pancreatic cancer; high expression of RBM3 was demonstrated to confer a more aggressive behavior through enhanced migration and invasion of the primary tumor, with increased sensitivity to adjuvant chemotherapy, particularly gemcitabine.
3.7. Other Cancers
One study suggested that RBM3 may be a potential biomarker for the stratification of patients with metastatic non-seminomatous germ cell tumors (NSGCT). Low RBM3 expression correlates with worse prognosis and an increased risk of treatment failure and high RBM3 expression is considered a predictor of enhanced cisplatin sensitivity in NSGCT [52]. Similar results have been reported for non-small cell lung cancer (NSCLC), in which the loss of RBM3 expression is related to aggressive tumor features and poor clinical outcomes in patients with LUAC (lung adenocarcinoma) [51]. These findings are consistent with those of a prospective population-based cohort study that considered RBM3 an independent biomarker of prolonged OS in patients with primary malignant melanomas and showed that RBM3 was downregulated during the progression of metastatic melanoma [78]. Moreover, researchers have found that RBM3 is upregulated in radioresistant nasopharyngeal carcinoma (NPC) tissues and cells, and RBM3 overexpression in NPC cells (CNE1) significantly drives radioresistance, inhibits DNA damage, and reduces the rate of apoptosis via the PI3K/AKT/Bcl-2 signaling pathway [54].
4. Discussion
RBM3 is an RNA-binding protein with multiple physiological functions. RBM3 is induced in response to various extracellular environmental conditions such as mild hypothermia, UV irradiation, nitric oxide stress, ER stress, hypoxic-ischemic stress, oxygen-glucose deprivation/reperfusion injury, inflammation, I/R-induced myocardial injury, aortic dissection, neurodegenerative disease, and different types of cancers. A previous study has shown that the transcription factor NF-κB p65 is phosphorylated at ser276 and activates RBM3 transcription by binding to a particular element within the promoter region [4]. Overall, RBM3 expression may be regulated not only by one transcription factor but also by multiple transcription factors. This requires further investigation. Although RBM3 is an RNA-binding protein, it has downstream RNAs, including mRNAs, lncRNAs, and miRNAs. Although some studies have been conducted, systematic studies that play an important role in revealing the functions of RBM3 are lacking.
One study showed that the blood samples of patients with post-cardiac arrest treated with TTM had significantly increased expression of RBM3 mRNA. However, the upregulation of RBM3 protein expression in response to TTM was not observed [2]. RBM3 is confirmed to be mainly expressed in the nucleus, and it has been found to shuttle between the nucleus and the cytoplasm in response to adverse conditions. However, it is unclear whether RBM3 is secreted into the extracellular environment, such as the circulatory system, or whether it can be measured in full blood samples. The mechanism underlying the active secretion of RBM3 and the role of extracellular RBM3 require further investigation, which is of great significance for a complete understanding of RBM3 functions.
RBM3 is upregulated in various cancers and is associated with clinical outcomes, indicating that RBM3 can be used as a diagnostic marker for cancer. RBM3 exerts different effects in different tumors, which is paradoxical to an extent. High expression of RBM3 promotes proliferation [20, 21, 47], promotes VEGF/IL-8 expression [18], inhibits apoptosis [23, 78], affects cancer cell stemness remodeling [24, 25, 46], and promotes DNA integrity [50, 54, 55]. The different biological functions of RBM3 in different cancers may be related to the heterogeneity and complexity of the tumors themselves. Detailed genotyping of tumors may help explain the different roles of RBM3 in different tumors. The expression levels of RBM3 in different cancers are inconsistent and are associated with different prognoses. RBM3, an RNA-binding protein, can exert different biological effects by interacting with different proteins in different types of tumors [1, 26, 27].
The tumor cell microenvironment plays a pivotal role in tumor cell activity, and temperature is a central component of the microenvironment. A study showed that downregulating RBM3 expression by exposure to heat treatment greatly impairs prostate cancer cell survival and enhances chemosensitivity [59]. The study further showed that RBM3 was significantly decreased in PCa cells that survived stressful environments, such as soft agar and osteogenic microenvironments [24, 25], with evidence indicating that restoring RBM3 expression can suppress stemness remodeling in prostate cancer [24, 25]. These studies suggest that RBM3 may play different roles in different microenvironments of cancer cells, such as hormone levels and immune cell infiltration. The mechanism by which RBM3 exerts its function in the tumor microenvironment requires further study. Differences at epigenetic levels may also affect the function of RBM3. In addition, RBM3 variants [33] or the type of protein modification may have an important influence on the function of RBM3, and the study of these aspects will be helpful in further elucidating the role of RBM3 in cancer.
We found that different levels of RBM3 expression in several types of cancer could enhance chemosensitivity [50, 52, 53, 59, 61]. ADDIN EN. CITE chemotherapy is believed to improve the immunogenicity of cancers [79]. Recent studies on antitumor therapy have found that increasing the immunogenicity of cold tumor cells transforms them into hot tumors, thereby improving the effectiveness of tumor immunotherapy [79-82]. As a stress-response protein, RBM3 mediates the response of PCa cells in the tumor microenvironment, and RBM3 suppresses stemness remodeling of prostate cancer in the bone microenvironment [25]. This epigenetic regulatory approach to RBM3 may be the key mechanism by which tumor cells adapt to changes in the microenvironment. Based on this, we conclude that RBM3 is probably a key molecule for turning cold tumors hot. However, this requires further research.
Nevertheless, it might be challenging to accurately determine the roles of RBM3, as contradictory results relative to specific types of cancer have been reported. There is no doubt that further study on the underlying regulatory mechanisms of the effect of RBM3 in oncogenesis or as a tumor suppressor is still greatly needed. Meanwhile, there is a need to evaluate the relationship between levels of RBM3 expression during different periods in numerous cancers to stratify patients for personalized prevention and therapy.
In this review, we discussed the essential roles and mechanisms of action of RBM3. Cold stress is a common physiological phenomenon and RBM3 is a cold shock protein that plays essential roles in cytoprotection, anti-apoptosis, cell survival, and neuroprotection. RBM3 participates in the development and progression of neurodegenerative diseases, such as alzheimer's disease. RBM3 mediates cold-induced structural plasticity, which is necessary for maintaining the synapse number [8]. Apam the functions mentioned above, accumulating evidence suggests that RBM3 plays essential roles in cancer.
5. Conclusion
RBM3 is an RNA-binding protein with multiple physiological functions that are closely linked to many biological processes beyond its role in cold stress. Additionally, RBM3 is a cold-shock protein whose expression is as protective as that of cooling. However, this aspect warrants further investigation.
Data Availability
All the data can be obtained by contacting the corresponding author when it’s necessary.
Conflicts of Interest
None.
Acknowledgments
This study is supported by Sichuan Science and Technology Program 2022YFS0632, and the joint foundation of Luzhou government and Southwest Medical University (2021LZXNYD-J28).
Author Contributions
Qiaoli Yuan wrote the first draft of the article, Xingchen Zhou conducted data collection and analysis, Lisirui Ge produced the figures and Xiang Haizhou revised the article. Jianguo Feng guided the writing of the paper. Maohua Wang designed this study and guided the writing of the article and the final revision of the manuscript.
REFERENCES
[1] Jessie M Sutherland, Nicole A
Siddall, Gary R Hime, et al. “RNA binding proteins in spermatogenesis: an in
depth focus on the Musashi family.” Asian J Androl, vol. 17, no. 4, pp.
529-536, 2015. View at: Publisher
Site | PubMed
[2] Lisa-Maria Rosenthal, Christoph
Leithner, Giang Tong, et al. “RBM3 and CIRP expressions in targeted temperature
management treated cardiac arrest patients-A prospective single center study.” PLoS
One, vol. 14, no. 12, pp. e0226005, 2019. View at: Publisher Site | PubMed
[3] Bingjin Liu, Yun Cao, Fangxiao Shi,
et al. “The overexpression of RBM3 alleviates TBI-induced behaviour impairment
and AD-like tauopathy in mice.” J Cell Mol Med, vol. 24, no. 16, pp.
9176-9188, 2020. View at: Publisher
Site | PubMed
[4] Ayako Ushio, Ko Eto “RBM3 expression
is upregulated by NF-κB p65 activity, protecting cells from apoptosis, during mild hypothermia.” J Cell Biochem, vol. 119, no.
7, pp. 5734-5749, 2018. View at: Publisher
Site | PubMed
[5] Kosuke Saito, Noboru Fukuda, Taro
Matsumoto, et al. “Moderate low temperature preserves the stemness of neural
stem cells and suppresses apoptosis of the cells via activation of the
cold-inducible RNA binding protein.” Brain Res, vol. 1358, pp. 20-29,
2010. View at: Publisher
Site | PubMed
[6] Hai-Jie Yang, Rui-Juan Zhuang,
Yuan-Bo Li, et al. “Cold‐inducible protein RBM3 mediates
hypothermic neuroprotection against neurotoxin rotenone via inhibition on MAPK
signalling.” J Cell Mol
Med, vol. 23, no. 10, pp. 7010-7020, 2019. View at: Publisher Site | PubMed
[7] Xinzhou Zhu, Jingyi Yan, Catherine
Bregere, et al. “RBM3 promotes neurogenesis in a niche-dependent manner via
IMP2-IGF2 signaling pathway after hypoxic-ischemic brain injury.” Nat Commun,
vol. 10, no. 1, pp. 3983, 2019. View at: Publisher Site | PubMed
[8] Diego Peretti, Amandine Bastide,
Helois Radford, et al. “RBM3 mediates structural plasticity and protective
effects of cooling in neurodegeneration.” Nature, vol. 518, no. 7538,
pp. 236-239, 2015. View at: Publisher
Site | PubMed
[9] Sophorn Chip, Andrea Zelmer, Omolara
O Ogunshola, et al. “The RNA-binding protein RBM3 is involved in hypothermia
induced neuroprotection.” Neurobiol Dis, vol. 43, no. 2, pp. 388-396,
2011. View at: Publisher
Site | PubMed
[10]
Xinzhou
Zhu, Andrea Zelmer, Josef P Kapfhammer, et al. “Cold-inducible RBM3 inhibits
PERK phosphorylation through cooperation with NF90 to protect cells from
endoplasmic reticulum stress.” FASEB J, vol. 30, no. 2, pp. 624-634,
2016. View at: Publisher
Site | PubMed
[11]
Sven
Wellmann, Matthias Truss, Elisabeth Bruder, et al. “The RNA-binding protein
RBM3 is required for cell proliferation and protects against serum
deprivation-induced cell death.” Pediatr Res, vol. 67, no. 1, pp. 35-41,
2010. View at: Publisher
Site | PubMed
[12]
Hongzhao
Shi, Ruizhi Yao, Shuai Lian, et al. “Regulating glycolysis, the TLR4 signal
pathway and expression of RBM3 in mouse liver in response to acute cold
exposure.” Stress, vol. 22, no. 3, pp. 366-376, 2019. View at: Publisher Site | PubMed
[13]
Hai-Jie
Yang, Fei Ju, Xin-Xin Guo, et al. “RNA-binding protein RBM3 prevents NO-induced
apoptosis in human neuroblastoma cells by modulating p38 signaling and
miR-143.” Sci Rep, vol. 7, pp. 41738, 2017. View at: Publisher Site | PubMed
[14]
Amy
L Ferry, Peter W Vanderklish, Esther E Dupont-Versteegden “Enhanced survival of
skeletal muscle myoblasts in response to overexpression of cold shock protein
RBM3.” Am J Physiol Cell Physiol, vol. 301, no. 2, pp. C392-C402, 2011.
View at: Publisher
Site | PubMed
[15]
Rui-Juan
Zhuang, Jian Ma, Xiang Shi, et al. “Cold-Inducible Protein RBM3 Protects UV
Irradiation-Induced Apoptosis in Neuroblastoma Cells by Affecting p38 and JNK
Pathways and Bcl2 Family Proteins.” J Mol Neurosci, vol. 63, no. 2, pp.
142-151, 2017. View at: Publisher
Site | PubMed
[16]
Wenwen
Si, Zhen Li, Zifeng Huang, et al. “RNA Binding Protein Motif 3 Inhibits
Oxygen-Glucose Deprivation/Reoxygenation-Induced Apoptosis Through Promoting
Stress Granules Formation in PC12 Cells and Rat Primary Cortical Neurons.” Front
Cell Neurosci, vol. 14, pp. 559384, 2020. View at: Publisher Site | PubMed
[17]
Yang
Liu, Hongzhao Shi, Yajie Hu, et al. “RNA binding motif protein 3 (RBM3)
promotes protein kinase B (AKT) activation to enhance glucose metabolism and
reduce apoptosis in skeletal muscle of mice under acute cold exposure.” Cell
Stress Chaperones, vol. 27, no. 6, pp. 603-618, 2022. View at: Publisher Site | PubMed
[18]
S
M Sureban, S Ramalingam, G Natarajan, et al. “Translation regulatory factor
RBM3 is a proto-oncogene that prevents mitotic catastrophe.” Oncogene,
vol. 27, no. 33, pp. 4544-4556, 2008. View at: Publisher Site | PubMed
[19]
Jingyi
Yan, Tessa Goerne, Andrea Zelmer, et al. “The RNA-Binding Protein RBM3 Promotes
Neural Stem Cell (NSC) Proliferation Under Hypoxia.” Front Cell Dev Biol,
vol. 7, pp. 288, 2019. View at: Publisher Site | PubMed
[20]
Ping
Chen, Xiaoli Yue, Hongbing Xiong, et al. “RBM3 upregulates ARPC2 by binding the
3'UTR and contributes to breast cancer progression.” Int J Oncol, vol.
54, no. 4, pp. 1387-1397, 2019. View at: Publisher Site | PubMed
[21]
Lu
Zhang, Yi Zhang, Dongliang Shen, et al. “RNA Binding Motif Protein 3 Promotes
Cell Metastasis and Epithelial-Mesenchymal Transition Through STAT3 Signaling
Pathway in Hepatocellular Carcinoma.” J Hepatocell Carcinoma, vol. 9,
pp. 405-422, 2022. View at: Publisher
Site | PubMed
[22]
Daniel
A Lujan, Joey L Ochoa, Rebecca S Hartley “Cold-inducible RNA binding protein in
cancer and inflammation.” Wiley Interdiscip Rev RNA, vol. 9, no. 2, pp.
10.1002/wrna.1462. View at: Publisher
Site | PubMed
[23]
Toshiharu
Sakurai, Hiroshi Kashida, Yoriaki Komeda, et al. “Stress Response Protein RBM3
Promotes the Development of Colitis-associated Cancer.” Inflamm Bowel Dis,
vol. 23, no. 1, pp. 57-65, 2017. View at: Publisher Site | PubMed
[24]
Yu
Zeng, Dana Wodzenski, Dong Gao, et al. “Stress-response protein RBM3 attenuates
the stem-like properties of prostate cancer cells by interfering with CD44
variant splicing.” Cancer Res, vol. 73, no. 13, pp. 4123-4133, 2013.
View at: Publisher
Site | PubMed
[25]
Shouyi
Zhang, Chengcheng Lv, Yichen Niu, et al. “RBM3 suppresses stemness remodeling
of prostate cancer in bone microenvironment by modulating N6-methyladenosine on
CTNNB1 mRNA.” Cell Death Dis, vol. 14, no.2, pp. 91, 2023. View at: Publisher Site | PubMed
[26]
Wenlong
Xia, Libo Su, Jianwei Jiao “Cold-induced protein RBM3 orchestrates neurogenesis
via modulating Yap mRNA stability in cold stress.” J Cell Biol, vol.
217, no. 10, pp. 3464-3479, 2018. View at: Publisher Site | PubMed
[27]
Mohamed
B Al-Fageeh, C Mark Smales “Cold-inducible RNA binding protein (CIRP)
expression is modulated by alternative mRNAs.” RNA, vol. 15, no. 6, pp.
1164-1176, 2009. View at: Publisher
Site | PubMed
[28]
Marta
Costa, Alessio Squassina, Ignazio Stefano Piras, et al. “Preliminary
Transcriptome Analysis in Lymphoblasts from Cluster Headache and Bipolar
Disorder Patients Implicates Dysregulation of Circadian and Serotonergic
Genes.” J Mol Neurosci, vol. 56, no. 3, pp. 688-695, 2015. View at: Publisher Site | PubMed
[29]
J
M Derry, J A Kerns, U Francke “RBM3, a novel human gene in Xp11.23 with a
putative.” Hum Mol Genet, vol. 4, no. 12, pp. 2307-2311, 1995. View at: Publisher Site | PubMed
[30]
Xinzhou
Zhu, Christoph Bührer, Sven Wellmann “Cold-inducible proteins CIRP and RBM3, a
unique couple with activities far beyond the cold.” Cell Mol Life Sci,
vol. 73, no. 20, pp. 3839-3859, 2016. View at: Publisher Site | PubMed
[31]
Sayantani
Roy, Soumendu Boral, Snigdha Maiti, et al. “Structural and dynamic studies of
the human RNA binding protein RBM3 reveals the molecular basis of its
oligomerization and RNA recognition.” FEBS J, vol. 289, no. 10, pp.
2847-2864, 2021. View at: Publisher
Site | PubMed
[32]
Bradley
M Lunde, Claire Moore, Gabriele Varani “RNA-binding proteins: modular design
for efficient function.” Nat Rev Mol Cell Biol, vol. 8, no. 6, pp.
479-490, 2007. View at: Publisher
Site | PubMed
[33]
Fiona
Smart, Armaz Aschrafi, Annette Atkins, et al. “Two isoforms of the
cold-inducible mRNA-binding protein RBM3 localize to dendrites and promote
translation.” J Neurochem, vol. 101, no. 5, pp. 1367-1379, 2007. View
at: Publisher
Site | PubMed
[34]
S
Danno, K Itoh, T Matsuda, et al. “Decreased Expression of Mouse Rbm3, a
Cold-Shock Protein, in Sertoli Cells of Cryptorchid Testis.” Am J Pathol,
vol. 156, no. 5, pp. 1685-1692, 2000. View at: Publisher Site | PubMed
[35]
John
Dresios, Armaz Aschrafi, Geoffrey C Owens, et al. “Cold stress-induced protein
Rbm3 binds 60S ribosomal subunits, alters microRNA levels,and enhances global
protein synthesis.” Proc Natl Acad Sci U S A, vol. 102, no. 6, pp.
1865-1870, 2005. View at: Publisher
Site | PubMed
[36]
Miguel
Perez-Pouchoulen, Ayodele Jaiyesimi, Keti Bardhi, et al. “Hypothermia increases
cold-inducible protein expression and improves cerebellar-dependent learning
after hypoxia ischemia in the neonatal rat.” Pediatr Res, vol. 94, no.
2, pp. 539-546, 2023. View at: Publisher Site | PubMed
[37]
Marco
Preußner, Heather L Smith, Daniel Hughes, et al. “ASO targeting RBM3
temperature-controlled poison exon splicing prevents neurodegeneration in
vivo.” EMBO Mol Med, vol. 15, no. 5, pp. e17157, 2023. View at: Publisher Site | PubMed
[38]
Junnosuke
Nakamura, Takuma Aihara, Tomoki Chiba, et al. “Cold shock protein RBM3 is
upregulated in the autophagy-deficient brain.” MicroPubl Biol, vol.
2022, pp. 10.17912/micropub.biology.000695, 2022. View at: Publisher Site | PubMed
[39]
Diego
Peretti, Heather L Smith, Nicholas Verity, et al. “TrkB signaling regulates the
cold-shock protein RBM3-mediated neuroprotection.” Life Sci Alliance,
vol. 4, no. 4, pp. e202000884, 2021. View at: Publisher Site | PubMed
[40]
Zachary
R Hettinger, Amy L Confides, Peter W Vanderklish, et al. “The transcript interactome
of skeletal muscle RNA binding protein motif 3 (RBM3).” Physiol Rep,
vol. 11, no. 3, pp. e15596, 2023. View at: Publisher Site | PubMed
[41]
Feiyu
Long, Liren Hu, Yingxu Chen, et al. “RBM3 is associated with acute lung injury
in septic mice and patients via the NF-kappaB/NLRP3 pathway.” Inflamm Res,
vol. 72, no. 4, pp. 731-744, 2023. View at: Publisher Site | PubMed
[42]
Nan
Wang, Limeiting Wang, Changyan Li, et al. “RBM3 interacts with Raptor to
regulate autophagy and protect cardiomyocytes from ischemia-reperfusion-induced
injury.” J Physiol Biochem, vol. 79, no. 1, pp. 47-57, 2023. View at: Publisher Site | PubMed
[43]
Yifan
Xie, Rifeng Gao, Yang Gao, et al. “Proteasome Activator REGγ Promotes Aortic Dissection by
Inhibiting RBM3 (RNA Binding Motif Protein 3) Pathway.” Hypertension, vol. 80, no. 1,
pp. 125-137, 2023. View at: Publisher
Site | PubMed
[44]
Justin
J-L Wong, Amy Y M Au, Dadi Gao, et al. “RBM3 regulates temperature sensitive
miR-142-5p and miR-143 (thermomiRs), which target immune genes and control
fever.” Nucleic Acids Res, vol. 44, no. 6, pp. 2888-2897, 2016. View at:
Publisher Site | PubMed
[45]
Jana
H Badrani, Allyssa N Strohm, Lee Lacasa, et al. “RNA-binding protein RBM3
intrinsically suppresses lung innate lymphoid cell activation and inflammation
partially through CysLT1R.” Nat Commun, vol. 13, no. 1, pp. 4435, 2022.
View at: Publisher
Site | PubMed
[46]
Anand
Venugopal, Dharmalingam Subramaniam, Julia Balmaceda, et al. “RNA binding
protein RBM3 increases beta-catenin signaling to increase stem cell
characteristics in colorectal cancer cells.” Mol Carcinog, vol. 55, no.
11, pp. 1503-1516, 2016. View at: Publisher
Site | PubMed
[47]
Wei
Dong, Zhi-Hui Dai, Fu-Chen Liu, et al. “The RNA-binding protein RBM3 promotes
cell proliferation in hepatocellular carcinoma by regulating circular RNA
SCD-circRNA 2 production.” EBioMedicine, vol. 45, pp. 155-167, 2019.
View at: Publisher
Site | PubMed
[48]
J
Pilotte, W Kiosses, S W Chan, et al. “Morphoregulatory functions of the
RNA-binding motif protein 3 in cell spreading, polarity and migration.” Sci
Rep, vol. 8, no. 1, pp. 7367, 2018. View at: Publisher Site | PubMed
[49]
Hyuna
Sung, Jacques Ferlay, Rebecca L Siegel, et al. “Global Cancer Statistics 2020:
GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185
Countries.” CA Cancer J Clin, vol. 71, no. 3, pp. 209-249, 2021. View
at: Publisher Site | PubMed
[50]
Asa
Ehlén, Donal J Brennan, Björn Nodin, et al. “Expression of the RNA-binding
protein RBM3 is associated with a favourable prognosis and cisplatin
sensitivity in epithelial ovarian cancer.” J Transl Med, vol. 8, pp. 78,
2010. View at: Publisher
Site | PubMed
[51]
Nathaniel
Melling, Kai Bachmann, Bianca Hofmann, et al. “Prevalence and clinical
significance of RBM3 immunostaining in non-small cell lung cancers.” J
Cancer Res Clin Oncol, vol. 145, no. 4, pp. 873-879, 2019. View at: Publisher Site | PubMed
[52]
Sven-Erik
Olofsson, Björn Nodin, Alexander Gaber, et al. “Low RBM3 protein expression
correlates with clinical stage, prognostic classification and increased risk of
treatment failure in testicular non-seminomatous germ cell cancer.” PLoS One,
vol. 10, no. 3, pp. e0121300, 2015. View at: Publisher Site | PubMed
[53]
Sara
Wahlin, Karolina Boman, Bruce Moran, et al. “Pre-clinical and clinical studies
on the role of RBM3 in muscle-invasive bladder cancer: longitudinal expression,
transcriptome-level effects and modulation of chemosensitivity.” BMC Cancer,
vol. 22, no. 1, pp. 131, 2022. View at: Publisher Site | PubMed
[54]
Rui
Ma, Li-Na Zhao, Hua Yang, et al. “RNA binding motif protein 3 (RBM3) drives
radioresistance in nasopharyngeal carcinoma by reducing apoptosis via the
PI3K/AKT/Bcl-2 signaling pathway.” Am J Transl Res, vol. 10, no. 12, pp.
4130-4140, 2018. View at: PubMed
[55]
Karolina
Boman, Gustav Andersson, Christoffer Wennersten, et al. “Podocalyxin-like and
RNA-binding motif protein 3 are prognostic biomarkers in urothelial bladder
cancer: a validatory study.” Biomark Res, vol. 5, pp. 10, 2017. View at:
Publisher Site | PubMed
[56]
Nathaniel
Melling, Ronald Simon, Martina Mirlacher, et al. “Loss of RNA-binding motif
protein 3 expression is associated with right-sided localization and poor
prognosis in colorectal cancer.” Histopathology, vol. 68, no. 2, pp.
191-198, 2016. View at: Publisher
Site | PubMed
[57]
Katharina
Grupp, Julia Wilking, Kristina Prien, et al. “High RNA-binding motif protein 3
expression is an independent prognostic marker in operated prostate cancer and
tightly linked to ERG activation and PTEN deletions.” Eur J Cancer, vol.
50, no. 4, pp. 852-861, 2014. View at: Publisher Site | PubMed
[58]
Liv
Jonsson, Alexander Gaber, David Ulmert, et al. “High RBM3 expression in
prostate cancer independently predicts a reduced risk of biochemical recurrence
and disease progression.” Diagn Pathol, vol. 6, pp. 91, 2011. View at: Publisher Site | PubMed
[59]
Yu
Zeng, Prakash Kulkarni, Takahiro Inoue, et al. “Down-regulating cold shock
protein genes impairs cancer cell survival and enhances chemosensitivity.” J
Cell Biochem, vol. 107, no. 1, pp. 179-188, 2009. View at: Publisher Site | PubMed
[60]
Annika
Jögi, Donal J Brennan, Lisa Rydén, et al. “Nuclear expression of the
RNA-binding protein RBM3 is associated with an improved clinical outcome in
breast cancer.” Mod Pathol, vol. 22, no. 12, pp. 1564-1574, 2009. View
at: Publisher
Site | PubMed
[61]
Emelie
Karnevi, Liv Ben Dror, Adil Mardinoglu, et al. “Translational study reveals a
two-faced role of RBM3 in pancreatic cancer and suggests its potential value as
a biomarker for improved patient stratification.” Oncotarget, vol. 9,
no. 5, pp. 6188-6200, 2017. View at: Publisher Site | PubMed
[62]
M
E Lleonart “A new generation of proto-oncogenes: cold-inducible RNA binding
proteins.” Biochim Biophys Acta, vol. 1805, no. 1, pp. 43-52, 2010. View
at: Publisher
Site | PubMed
[63]
Åsa
Ehlén, Björn Nodin, Elton Rexhepaj, et al. “RBM3-regulated genes promote DNA
integrity and affect clinical outcome in epithelial ovarian cancer.” Transl
Oncol, vol. 4, no. 4, pp. 212-221, 2011. View at: Publisher Site | PubMed
[64]
Seong-Hwan
Park, Yuseok Moon “Integrated stress response-altered pro-inflammatory signals
in mucosal immune-related cells.” Immunopharmacol Immunotoxicol, vol.
35, no. 2, pp. 205-214, 2013. View at: Publisher Site | PubMed
[65]
M
Hockel, K Schlenger, B Aral, et al. “Association between tumor hypoxia and
malignant progression in advanced cancer of the uterine cervix.” Cancer Res,
vol. 56, no. 19, pp. 4509-4515, 1996. View at: PubMed
[66]
Adrian
L Harris “Hypoxia -- a key regulatory factor in tumour growth.” Nat Rev
Cancer, vol. 2, no. 1, pp. 38-47, 2002. View at: Publisher Site | PubMed
[67]
John
M Heddleston, Zhizhong Li, Roger E McLendon, et al. “The hypoxic
microenvironment maintains glioblastoma stem cells and promotes reprogramming
towards a cancer stem cell phenotype.” Cell Cycle, vol. 8, no. 20, pp.
3274-3284, 2009. View at: Publisher
Site | PubMed
[68]
Qingzhuo
Dong, Chengcheng Lv, Gejun Zhang, et al. “Impact of RNA‑binding motif 3 expression on the whole transcriptome of prostate cancer
cells: An RNA sequencing study.” Oncol Rep, vol. 40, no. 4, pp. 2307-2315, 2018. View at: Publisher Site | PubMed
[69]
Remond
J A Fijneman, Robert T Cormier “The roles of sPLA2-IIA (Pla2g2a) in cancer of
the small and large intestine.” Front Biosci, vol. 13, pp. 4144-4174,
2008. View at: Publisher Site | PubMed
[70]
Michael
S Kuefner, Kevin Pham, Jeanna R Redd, et al. “Secretory phospholipase A group
IIA modulates insulin sensitivity and metabolism.” J Lipid Res, vol. 58,
no. 9, pp. 1822-1833, 2017. View at: Publisher Site | PubMed
[71]
Jun
Zhang, Yi Liu, Chang-Jun Yu, et al. “Role of ARPC2 in Human Gastric Cancer.” Mediators
Inflamm, vol. 2017, pp. 5432818, 2017. View at: Publisher Site | PubMed
[72]
Fernando
Martínez-Arribas, David Agudo, Marina Pollán, et al. “Positive correlation
between the expression of X-chromosome RBM genes (RBMX, RBM3, RBM10) and the
proapoptotic Bax gene in human breast cancer.” J Cell Biochem,
vol. 97, no. 6, pp. 1275-1782, 2006. View at: Publisher Site | PubMed
[73]
Stephanie
Lheureux, Marsela Braunstein, Amit M Oza “Epithelial ovarian cancer: Evolution
of management in the era of precision medicine.” CA Cancer J Clin, vol.
69, no. 4, pp. 280-304, 2019. View at: Publisher Site | PubMed
[74]
Stephanie
Lheureux, Charlie Gourley, Ignace Vergote, et al. “Epithelial ovarian cancer.” Lancet,
vol. 393, no. 10177, pp. 1240-1253, 2019. View at: Publisher Site | PubMed
[75]
Maximilian
Burger, James W F Catto, Guido Dalbagni, et al. “Epidemiology and risk factors
of urothelial bladder cancer.” Eur Urol, vol. 63, no. 2, pp. 234-241,
2013. View at: Publisher
Site | PubMed
[76]
Vicenç
Ruiz de Porras, Juan Carlos Pardo, Olatz Etxaniz, et al. “Neoadjuvant therapy
for muscle-invasive bladder cancer: Current clinical scenario, future
perspectives, and unsolved questions.” Crit Rev Oncol Hematol, vol. 178,
pp. 103795, 2022. View at: Publisher Site | PubMed
[77]
Liv
Jonsson, Charlotta Hedner, Alexander Gaber, et al. “High expression of
RNA-binding motif protein 3 in esophageal and gastric adenocarcinoma correlates
with intestinal metaplasia-associated tumours and independently predicts a
reduced risk of recurrence and death.” Biomark Res, vol. 2, pp. 11,
2014. View at: Publisher
Site | PubMed
[78]
Liv
Jonsson, Julia Bergman, Björn Nodin, et al. “Low RBM3 protein expression
correlates with tumour progression and poor prognosis in malignant melanoma: an
analysis of 215 cases from the Malmo Diet and Cancer Study.” J Transl Med,
vol. 9, pp. 114, 2011. View at: Publisher Site | PubMed
[79]
Jiahui
Zhang, Di Huang, Phei Er Saw, et al. Turning cold tumors hot: from molecular
mechanisms to clinical applications. Trends Immunol, vol. 43, no. 7, pp.
523-545, 2022. View at: Publisher
Site | PubMed
[80]
Wenjun
Xiong, Xueliang Gao, Tiantian Zhang, et al. “USP8 inhibition reshapes an
inflamed tumor microenvironment that potentiates the immunotherapy.” Nat
Commun, vol. 13, no. 1, pp. 1700, 2022. View at: Publisher Site | PubMed
[81] Youqian Wu, Chao Zhang, Xiaolan Liu, et al. “ARIH1 signaling promotes anti-tumor immunity by targeting PD-L1 for proteasomal degradation.” Nat Commun, vol. 12, no. 1, pp. 2346, 2021. View at: Publisher Site | PubMed
[82] Jérôme Galon, “Daniela Bruni Approaches to treat immune hot, altered and cold tumours with combination immunotherapies.” Nat Rev Drug Discov, vol. 18, no. 3, pp. 197-218, 2019. View at: Publisher Site | PubMed
