Received: Sat 23, Sep 2023
Accepted: Thu 12, Oct 2023
Abstract
A marginal ulcer is a commonly occurring complication of Roux-en-Y gastric bypass, with an occurrence rate of up to 16%. However, perforation of such ulcers is rare, affecting approximately 1% of cases and typically manifesting at a later stage. Moreover, risk factors, whether surgical or non-surgical, are present in nearly all patients reported in the literature, with only 0.2% found to have none. This case report focuses on a 32-year-old female patient who presented with severe left-sided abdominal pain. She had a history of undergoing Roux-en-Y surgery two years prior. A CT scan of the abdomen revealed the presence of free air and contrast leak, indicative of a perforated marginal ulcer. The patient subsequently underwent a laparoscopic repair of the marginal ulcer, involving the use of an omental patch. In patients experiencing recurrent abdominal pain, the suspicion of a marginal ulcer should arise, prompting the inclusion of upper endoscopy as part of the diagnostic workup. The feasibility of laparoscopic repair with an omental patch has been demonstrated, and patients are advised to undergo follow-up endoscopy. Doctors should have a high suspicion index for perforation of marginal ulcer with any patient after bariatric surgery despite the absence of risk factors.
Keywords
Perforated marginal ulcer, Roux-en-Y gastric bypass, RYGB, omental patch, bariatric complications, case report
1. Introduction
Stomal ulceration, also referred to as a marginal ulcer (MU), is a recognized complication following Roux-en-Y gastric bypass (RYGB) surgery, with reported incidence rates ranging from 0.6% to 16%. Nevertheless, research has revealed varying rates of perforation, with some studies indicating an incidence of over 1%, while others have reported rates between 1% and 2%. Notably, only 0.2% of patients were identified without associated risk factors [1-6]. Furthermore, a single case report described an instance of perforated marginal ulcer (PMU) occurring without identifiable risk factors four months post RYGB surgery [7]. The majority of PMUs tend to manifest around the 18-month mark, with rare occurrences at the 24-month interval or even as late as seven years [3, 4, 8]. This case report will discuss a rare occurrence of marginal ulcer perforation with no previous identifiable risk factor.
Identified risk factors for post-gastric bypass MU encompass smoking, preoperative NSAID usage, immunosuppression, colonization by Helicobacter pylori (H. pylori), trauma, foreign body ingestion, Crohn's disease, typhoid, tuberculosis, and malignancy. Research has discerned a correlation between escalating smoking intensity and heightened MU risk [7, 9, 10]. Additionally, literature has highlighted that larger anastomosis pouches can potentially contribute to the risk of marginal ulceration following RYGB [11]. The prevailing approach for PMU repair frequently involves suturing the ulcer, with or without the use of an omental patch, or revising the Roux-en-Y anastomosis. Notably, the same study has demonstrated that anastomosis revision carries a reduced risk of recurrence [12, 13].
2. Case Presentation
2.1. Patient Information and Past Medical History
A 32-year-old female was referred to our emergency department due to severe, sudden left-sided abdominal pain that had been present for one day. The pain was accompanied by nausea, two episodes of vomiting, and diarrhea. There was no fever or urinary symptoms. Initially, she sought care at a different hospital, where a contrast-free CT scan revealed the presence of free air that went unnoticed and unreported. Following this, another CT scan was conducted, this time with both oral and IV contrast, which documented the free air and contrast leak. It was only after this second scan that the patient was referred to our facility. She had a history of undergoing RYGB two years prior and had also undergone laparoscopic cholecystectomy in October 2022 without any documented complications. Her medical history was unremarkable apart from morbid obesity, and the patient denied any history of smoking, alcohol consumption, or use of NSAIDs or immunosuppression drugs. Notably, her preoperative weight (prior to the gastric bypass) was 106 kg, whereas her current weight was 55 kg. Additionally, the patient had undergone an H. pylori test before the RYGB surgery, which yielded negative results, although it is unclear whether she had been on proton pump inhibitors (PPI) treatment at that time.
2.2. Clinical Features and Examination Findings
Upon admission, the patient's vital signs were stable, with a pulse rate of 87 bpm. Her current body mass index (BMI) was 21 kg/m². Abdominal examination revealed tenderness on the left side of the abdomen, along with mild tenderness in the epigastric and umbilical areas. There was no guarding or rigidity, and bowel sounds were audible.
2.3. Laboratory and Radiological Findings
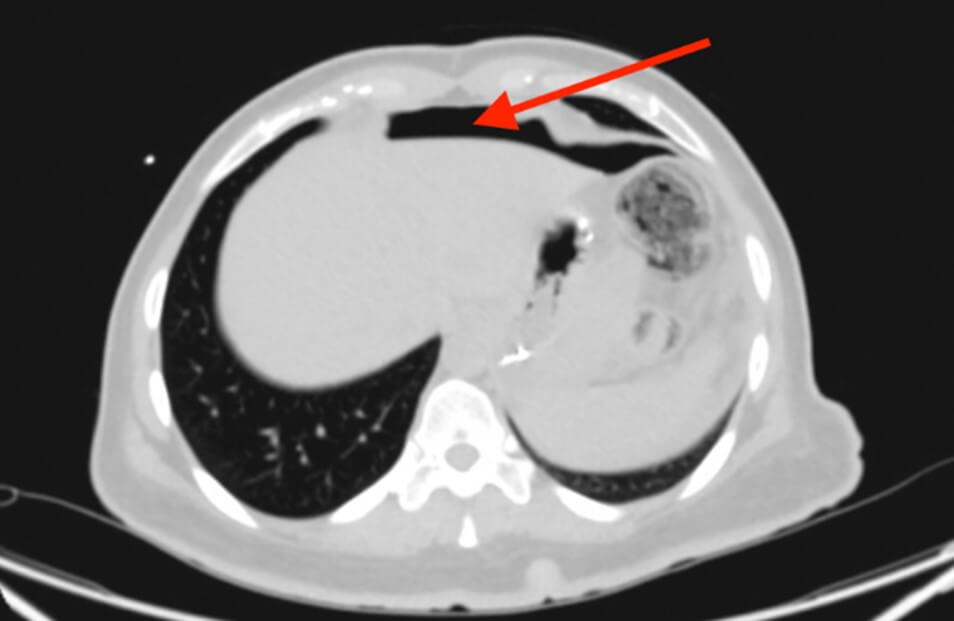
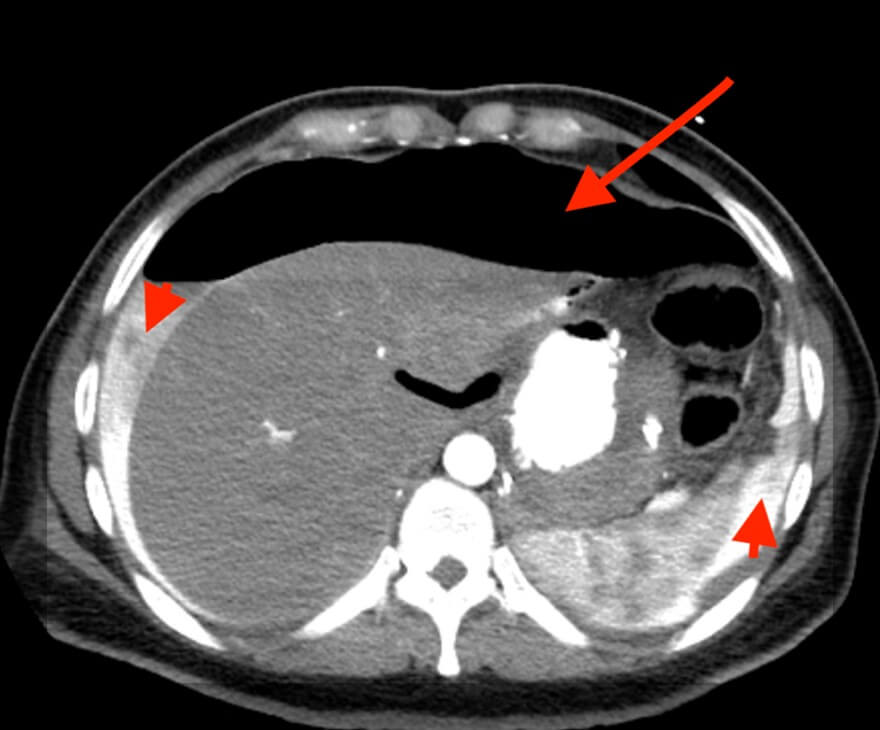
Upon presentation, her laboratory investigations indicated a white blood cell count (WBC) of 6.12 and a C-reactive protein (CRP) level of <0.5, while the remaining test results were within normal limits. Furthermore, an initial computed tomography (CT) scan of the abdomen without contrast (Figure 1) revealed a missed pneumoperitoneum but did show mild lower abdominal and pelvic collection. Subsequently, a CT scan of the abdomen with oral and IV contrast (Figure 2) was performed, revealing a large hydro-pneumo-peritoneum with a fluid level visible in the upper abdomen. The scan also indicated the presence of large intraperitoneal air loculi, suggesting leakage of oral contrast outside the gastrointestinal tract and into the intraperitoneal cavity surrounding the liver and spleen. Mild thickening of the wall of the jejunal loops was noted, along with a moderate amount of free fluid in the right iliac fossa and pelvic cavity.
2.4. Management: Operation with Endoscopy and Intraoperative Findings
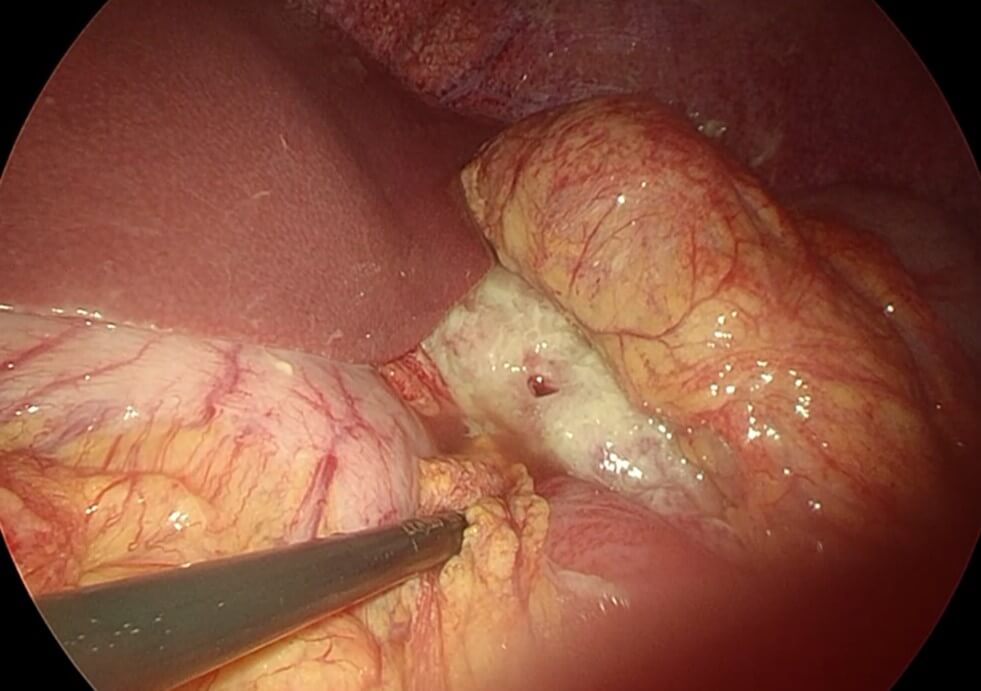
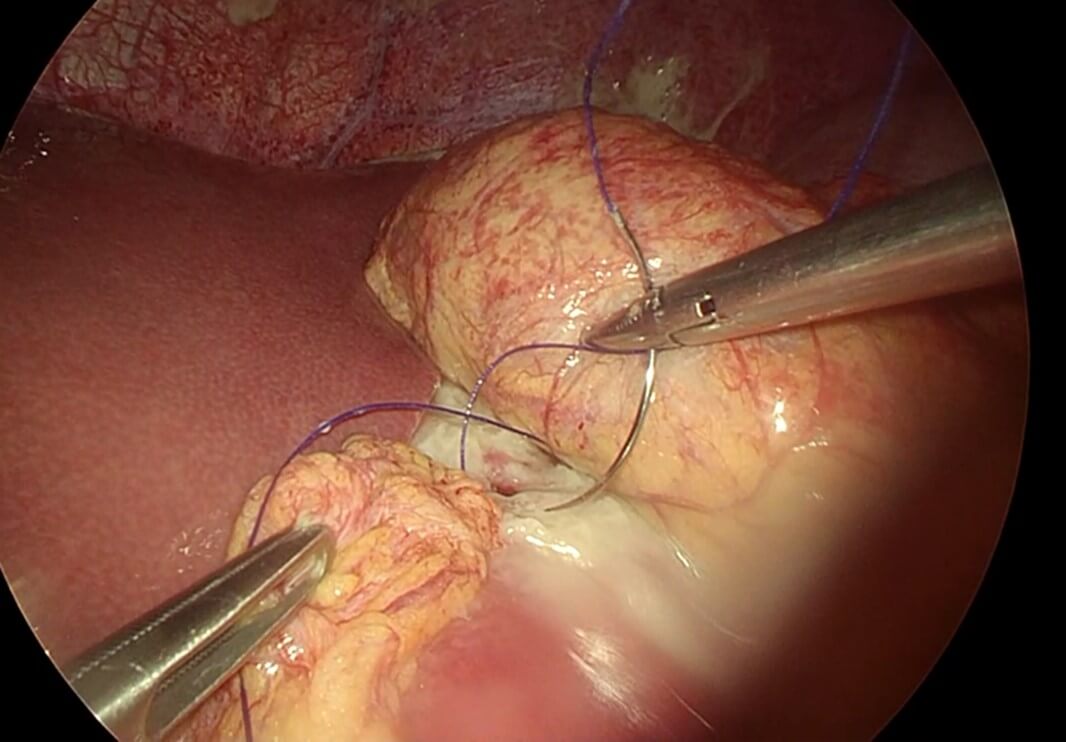
After proper resuscitation with intravenous fluids and intravenous antibiotics the patient was taken to the operative theatre for diagnostic laparoscopy and possible repair of the perforated marginal ulcer with intraoperative endoscopy (Video 1). Intraoperatively there was obvious contamination of the abdominal cavity with intestinal fluid and contrast material and there was evidence of fibrinous tissue covering the upper part of the remnant stomach, liver and small intestine with copious amount of gastric contents in the abdominal cavity (Figure 3), suctioning and washout of abdominal cavity was done and examination of the gastric pouch at the site of the anastomosis with the small intestine to create the roux limb there was a small perforation at the gastrointestinal anastomosis which was sutured with double stitch recreating 3-0 and omental fat attached to the suture (Figure 4). Examination of the whole small bowel showed no other injury and there is no internal hernia detected. Upper endoscopy was performed and it showed normal gastric pouch status post RYGB and there is ulcer seen at the small intestinal site just below the stoma with no evidence of air leak during the endoscopy. Finally, the abdomen was washed out with 3 liters of normal saline. The operation was uneventful and no complications took place intraoperatively nor early postoperatively.
2.5. Outcome and Follow-Up
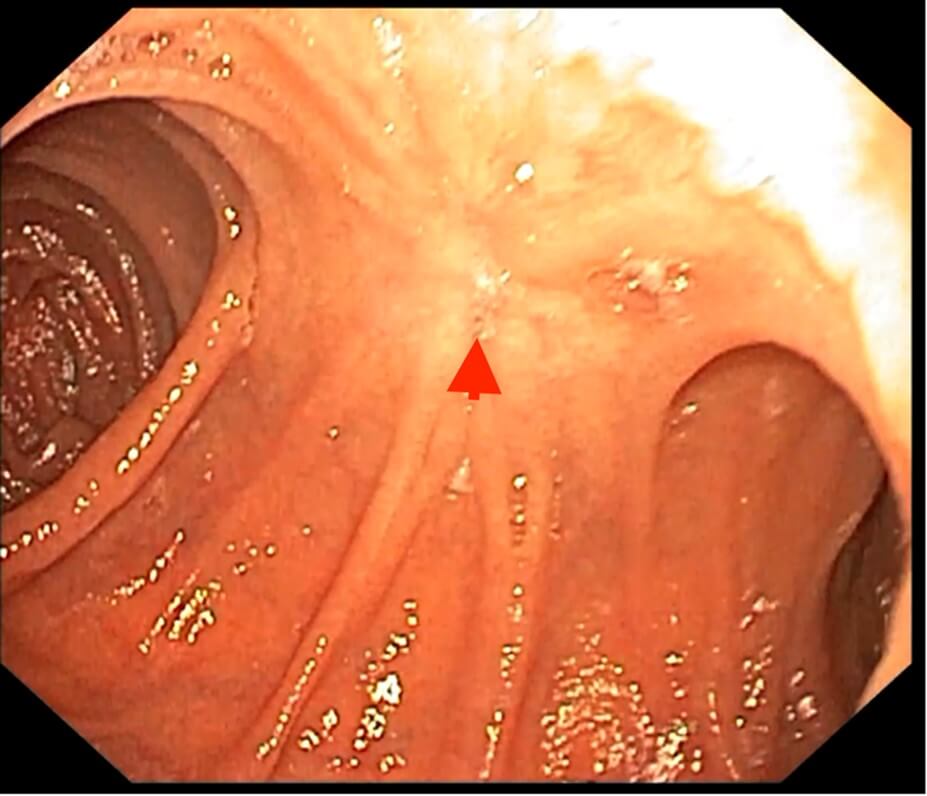
Post-operatively the patient was discharged after 3 days with no further complaints. Follow-up endoscopy was done after 4 weeks which showed healed ulcer with no evidence of stenosis or inflammation (Figure 5). Furthermore, H. pylori IgG and IgM test were done which revealed negative results. No biopsy was taken during the endoscopy and the patient was on PPI and sucralfate during the procedure. Gastrin level was 26 which was within the normal range.
3. Discussion
Roux-en-y is looked up as the gold-standard operation for weight loss and it's the most performed bariatric procedure. It provides around 65% weight loss by restriction and malabsorption [14]. The minor and major complication rates of the procedure were 27% and 3.3% respectively and its pooled mortality rate is 0.09% [15, 16]. The incidence of marginal ulcer is 0.6% to 16% and its perforation rate is 0.4%-1.0% and in a case series found to be 1% [14, 17, 18]. Marginal ulcer forms at the gastro-jejunal anastomosis and can present early (30 days or less) however perforation presents on average of 18 months (3-70 month range). The case presented after 24 months which coincides with other studies [3, 17]. Here we discuss a case of perforated marginal ulcer with no identified risk factors which is unusual and no such cases reported in the current literature.
Marginal ulcer risk factors can be divided into surgical and non-surgical. The non-surgical risk factors that are identified until date are; smoking, NSAIDs use pre- RYGB surgery, H. pylori infection, GERD, immunosuppression, corticosteroid use, and alcoholic consumption. In addition, one study found that preoperative hypertension was a significant predictor for marginal ulcer development. The surgical reasons identified so far are high gastric acidity in gastric pouch, ischemia of vessels, anastomotic tension, nonabsorbable sutures of the anastomosis, and the size of the gastric pouch which is proportionately related to marginal ulcer development [10, 14, 19-22]. However in relation to our case report, we could not identify any risk factor as to why the patient had a perforated marginal ulcer, even if we wanted to look at the surgical causes such as the anastomotic pouch size, in the endoscopy it was found to be of reasonable size, Thus this case had no previous and current risk factors.
Identifying a predisposing risk factor which has caused the perforation of the marginal ulcer in our patient is tricky. However, discussing the fact that H. pylori test that was done preoperatively could be a false negative is important here as it was noted in a study evaluating the possibility of a false negative of an H. pylori test was found that 56% of cases studied had a chance of unreliable false negative as they were on PPI during the esophagoduodenoscopy where the biopsies were taken [23]. Also a study noted that the incidence risk of H. pylori is high in patients who develop marginal ulcer postoperatively and this incidence risk is unchanged after eradication therapy [14]. Perforation was diagnosed on CT when free air was seen along with contrast leak. Then she underwent laparoscopic repair where an anterior perforated marginal ulcer was seen and it was repaired with omental fat and absence of leak was confirmed with the use of upper endoscopy in conjunction with the laparoscopy. Omental patch repair had shorter operative time, less blood loss and shorter hospital stay compared to other approaches [24, 25].
4. Conclusion
In the current literature we found one similar reported cases of a perforated marginal ulcer without an identified risk factor and however similar studies are needed to look for the pattern. It's important to note that there is a chance of a false negative H. pylori test as she was on PPI during the time of test. Thus, to increase the reliability of the test, the patient should not be on any PPI, antacids, or antibiotic therapy.
Patient Perspective
“The pain started suddenly yesterday and it was very severe, so I went to a hospital which has later sent me here. I had two keyhole surgeries, first to reduce my weight 2 years ago and the second was to remove my gallbladder less than a year ago. I was surprised when I heard that I need another keyhole surgery. However, I have a decent knowledge of what to expect after the surgery. After the operation, I did have some pain but it was night and day difference from what I had before it. A month after my surgery, I had to go for endoscopy to see the result and see if it is all good, and I was happy to hear that everything is healing well and that I don’t need to worry anymore.”
Consent for Publication
Verbal and written consents were obtained from the patient to publish the text, figures, and video of this manuscript, with anonymity maintained.
Standards of Reporting
SCARE guidelines were followed.
Conflicts of Interest
None.
Funding
None.
Acknowledgments
None.
Supplementary Materials
A (Video 1) of the omental patch repair operation is available for supplementary use only.
REFERENCES
[1] Alexander J Adduci, Catherine H
Phillips, Howard Harvin “Prospective diagnosis of marginal ulceration following
Roux-en-Y gastric bypass with computed tomography.” Radiol Case Rep,
vol. 10, no. 2, pp. 1063, 2016. View at: Publisher
Site | PubMed
[2] Madan
Haravu Srikantegowda, Ravi kumar Teppa, Vinay Kumar Salvadi, et al.
“Perforation of Gastrojejunal Anastomotic stomal ulcer- a rare delayed post
operative complication.” Int J Surg Med, vol. 8, no. 2, pp. 67-69, 2022. View at: Publisher Site
[3] Edward L Felix, John Kettelle, Elijah
Mobley, et al. “Perforated marginal ulcers after laparoscopic gastric bypass.” Surg
Endosc, vol. 22, no. 10, pp. 2128-2132, 2008. View at: Publisher Site | PubMed
[4] Brook
E. Porter, Lindsey E. Goldstein, Jeffrey E. Friedman “Marginal ulcer
perforation and concurrent 360-degree twisted roux limb volvulus following
roux-en-Y gastric bypass: A case report.” Bariatric Times, vol. 16, no.
6, pp. 16-17, 2019.
[5] Usha K Coblijn, Sjoerd M Lagarde,
Steve M M de Castro, et al. “Symptomatic marginal ulcer disease after roux-en-Y
gastric bypass: Incidence, risk factors and management.” Obes Surg, vol.
25, no. 5, pp. 805-811, 2015. View at: Publisher
Site | PubMed
[6] Maria
S Altieri, Aurora Pryor, Jie Yang, et al. “The natural history of perforated
marginal ulcers after gastric bypass surgery.” Surg Endosc, vol. 32, no.
3, pp. 1215-1222. View at: Publisher Site | PubMed
[7] Luke
D Fairweather, Toan D Pham “Non-marginal jejunal ulcer perforation following
Roux-en-Y gastric bypass.” J Surg Case Rep, vol. 2022, no. 3, pp.
rjac112. View at: Publisher Site | PubMed
[8] Yesenia
Brito, Omobolanle Kayode, Dominique Peters, et al. “Repair of a perforated
marginal ulcer seven years after roux-en y gastric bypass: A case report and
review of literature.” Cureus, vol. 15, no. 6, pp. e40750, 2023. View at: Publisher
Site | PubMed
[9] Adam Di Palma, Benjamin Liu, Azusa
Maeda, et al. “Marginal ulceration following Roux-en-Y gastric bypass: risk
factors for ulcer development, recurrence and need for revisional surgery.” Surg
Endosc, vol. 35, no. 5, pp. 2347-2353, 2021. View at: Publisher Site | PubMed
[10]
Luca
Dittrich, Marie-Valerie Schwenninger, Klaus Dittrich, et al. “Marginal ulcers
after laparoscopic Roux-en-Y gastric bypass: analysis of the amount of daily
and lifetime smoking on postoperative risk.” Surg Obes Relat Dis, vol.
16, no. 3, pp. 389-396, 2020. View at: Publisher
Site | PubMed
[11]
Kamal
Mahawar, Alistair J Sharples, Yitka Graham “A systematic review of the effect
of gastric pouch and/or gastrojejunostomy (stoma) size on weight loss outcomes
with Roux-en-Y gastric bypass.” Surg Endosc, vol. 34, no. 3, pp.
1048-1060, 2020. View
at: Publisher
Site | PubMed
[12]
Christopher
B Crawford, Leslie M Schuh, Margaret M Inma “Revision Gastrojejunostomy Versus
Suturing With and Without Omental Patch for Perforated Marginal Ulcer Treatment
After Roux-en-Y Gastric Bypass.” J Gastrointest Surg, vol. 27,
no. 1, pp. 1-6, 2023. View at: Publisher
Site | PubMed
[13]
Diego
Paim Carvalho Garcia, Cyntia Ferreira Dos Reis, Luiza Ohasi de Figueiredo, et
al. “Perforated gastric ulcer post mini gastric bypass treated by laparoscopy:
A case report.” Ann Med Surg (Lond), vol. 49, pp. 24-27, 2019. View at: Publisher Site | PubMed
[14]
Frederick Tiesenga, Luis F Adorno,
Datiobong Udoeyop, et al. “Perforated marginal ulcer.” Cureus, vol. 15,
no. 4, pp. e38127, 2023. View at: Publisher Site | PubMed
[15]
P
R Schauer, S Ikramuddin, W Gourash, et al. “Outcomes after laparoscopic
roux-en-Y gastric bypass for morbid obesity.” Ann Surg, vol. 232, no. 4,
pp. 515-529, 2000. View
at: Publisher
Site | PubMed
[16]
A
G N Robertson, T Wiggins, F P Robertson, et al. “Perioperative mortality in
bariatric surgery: Meta-analysis.” Br J Surg, vol. 108, no. 8, pp.
892-897, 2021. View
at: Publisher Site | PubMed
[17]
Basim
Alkhafaji, Muhammad Umar Younis, Yousif Al Khafaji “Laparoscopic repair of
perforated marginal ulcer after roux-en-Y gastric bypass: A case report and
review of literature.” Journal of Minimally Invasive Surgical Sciences,
pp. e84181, 2019. View
at: Publisher
Site
[18]
Edward
Wang, Ruth Blackham, Jeremy Tan, et al. “Giant perforated marginal ulcer after
laparoscopicroux-en-Y gastric bypass.” BMJ Case Rep, vol. 2017,
pp. bcr2016218829, 2017. View at: Publisher
Site | PubMed
[19]
K
J Printen, D Scott, E E Mason “Stomal ulcers after Gastric Bypass.” Arch
Surg, vol. 115, no. 4, pp. 525-527, 1980. View at: Publisher
Site | PubMed
[20]
Matthew
Wynn, Kristen M Tecson, David Provost “Marginal ulcers and associated risk
factors after roux-en-Y gastric bypass.” Proc (Bayl Univ Med Cent), vol.
36, no. 2, pp. 171-177, 2022. View at: Publisher Site | PubMed
[21]
Neil
H Bhayani, Tolulope A Oyetunji, David C Chang, et al. “Predictors of marginal
ulcers after laparoscopic roux-en-Y gastric bypass.” J Surg Res, vol.
177, no. 2, pp. 224-227, 2012. View at: Publisher
Site | PubMed
[22]
Sullivan
A Ayuso 1, Jordan N Robinson 2, Leslie M Okorji, et al. “Why size matters: An
evaluation of gastric pouch size in roux-en-Y gastric bypass using CT
volumetric analysis and its effect on marginal ulceration.” Obes Surg,
vol. 32, no. 3, pp. 587-592, 2022. View at: Publisher
Site | PubMed
[23]
Dor
Shirin, Shay Matalon, Benjamin Avidan, et al. “Real-world Helicobacter
pylori diagnosis in patients referred for esophagoduodenoscopy: The gap
between guidelines and clinical practice.” United European Gastroenterol J,
vol. 4, no. 6, pp. 762-769, 2016. View at: Publisher
Site | PubMed
[24] Mark R Wendling, John G Linn, Kara M Keplinger, et al. “Omental patch repair effectively treats perforated marginal ulcer following roux-en-Y gastric bypass.” Surg Endosc, vol. 27, no. 2, pp. 384-389, 2013. View at: Publisher Site | PubMed
[25] Ramya Kalaiselvan, Georgios Exarchos, Numan Hamza, et al. “Incidence of perforated gastrojejunal anastomotic ulcers after laparoscopic gastric bypass for morbid obesity and role of laparoscopy in their management.” Surg Obes Relat Dis, vol. 8, no. 4, pp. 423-428, 2012. View at: Publisher Site | PubMed
