Received: Mon 07, Aug 2023
Accepted: Sat 26, Aug 2023
Abstract
Background: Chronic kidney disease (CKD) and end-stage renal disease (ESRD) are emerging as a major health related issues in developing countries. The increasing numbers require rapid adaptation of existing health-care policies. The number of patients requiring renal care is only bound to increase in coming years with the increasing incidence and prevalence of diabetes. Continuous ambulatory peritoneal dialysis (CAPD) catheter placement is performed on end-stage renal disease (ESRD) patients. There is abundant literature regarding post-operative outcomes and re-operative rates following CAPD. We report our initial experience of modified single port laparoscopic technique of placing CAPD catheters using a single port through the Palmer’s point and discuss our surgical outcomes. Methods: This was a retrospective review (2019-2020) of patients undergoing CAPD catheter placement at SMVD Narayana Superspecialty Hospital. Outcome analysis focused on patient related outcomes, including early (< 30 days) versus late (≥ 30 days) complication and re-operation rates. Results: A total of 30 patients with ESRD, (mean ASA score=3.3) were included in the study who underwent modified single port laparoscopic (n=30) CAPD catheter placement (mean follow-up=180 days). The total complication rate with the procedure was 43%, with re-operation rate of 20%. CAPD catheter non-function occurred in 06 patients (20% of total). CAPD catheter migrations occurred in 03 patients (10% of total). CAPD catheter related infections occurred in 05 patients (16% of total), and 02 required re-operation; 03 patients were treated successfully with culture directed antibiotics. There were 2 deaths during the study period, one due to peritonitis and other due to underlying cardiac disease. There was no surgical mortality in the study group. Conclusion: Although CAPD catheter placement in patients with ESRD are technically un-complicated to accomplish, long term results suggest as many as one in three patients will struggle with some form of catheter malfunction or infection. Our modified technique and the results have led to changes in our CAPD catheter placement technique, as well as the post-operative patient care algorithm.
Keywords
CKD, peritoneal dialysis, CAPD, laparoscopy
1. Introduction
Laparoscopic assisted PD catheter placement was first described in the early 1990s. The safety and feasibility of laparoscopy has been referenced in many case reports, case series, comparative studies & retrospective reviews [1, 2]. With time the technique was refined and according to CMS data, laparoscopic guided PD catheter insertions is currently used in about 50 % of cases. Laparoscopy gives a good inspection of the ventral wall and inguinal areas to detect and treat occult abdominal wall hernias, intra-abdominal adhesions, omental bands, bowel laxity & redundancy to overcome postoperative catheter dysfunction.
2. Materials and Methods
2.1. Study Design
A retrospective analysis of database of all patients who underwent laparoscopic catheter placement from June 2019 to December 2020 at Shri Mata Vaishno Devi Superspeciality Hospital, Katra was performed. The study protocol was reviewed and approved by the institutional review board. All patients signed an informed consent form for catheter insertion. All procedures were performed laparoscopically and under anesthesia. 30 patients were included. There were no exclusion criteria, all the PD catheters was placed in an elective setting. Post operative course was monitored and medical records were analyzed for any complications. Catheter dislocation was confirmed with abdominal X-ray. Peritonitis was defined as clinical complaints of abdominal pain, cloudy dialysate, and leukocyte count greater than 100 cells/μL with more than 50% polymorphonuclear cells.
2.2. Study Population
ESRD patients following nephrology department on maintenance HD, previous PD and de-novo presentation were included in the study. The patients were counselled regarding different treatment options and possible complications of PD.
2.3. Technique Description
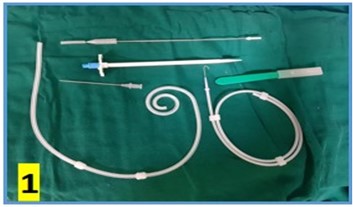
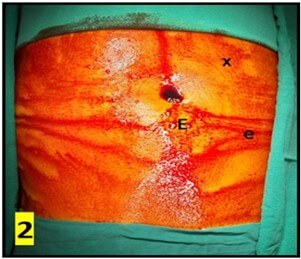
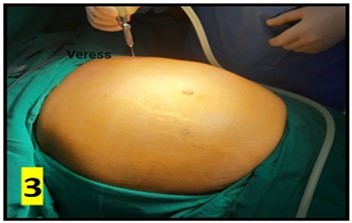
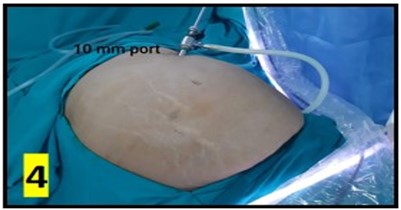
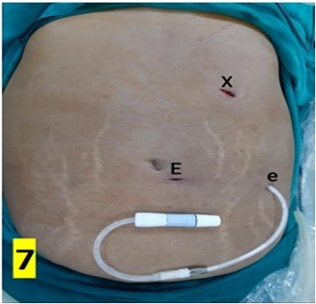
The CAPD catheter used was a straight, double-cuff, 42 cm tenckhoff catheter (Figure 1). All patients were admitted one day prior to the procedure. Pre-anesthesia check and baseline investigations were done. All patients received standard pre-operative preparation for laparoscopic surgery. Patient was kept fasting for 6 hours prior to procedure and were encouraged to empty bladder prior to shifting to OR. Belt-line location was marked (Figure 2). Patients received antibiotic prophylaxis (Inj. Vancomycin 500 mg) prior to surgery. Patients were positioned in supine position. 25 patients underwent the procedure under GA and 5 patients under SA. Average procedure time was 40 min (15-160 min). Pneumoperitoneum was created by veress technique through Palmer’s point (Figure 3). 10 mm port was placed through the Palmer’s point (Figure 4). Peritoneoscopy performed through 10mm port using 30 degree laparoscope (Figure 5) Tenckhoff catheter was placed under laparoscopic view at an infra-umbilical site using seldinger technique (Figure 6). With the patient in the supine position, the location of deep cuff was established by aligning the catheter tip with the upper border of the pubic symphysis and by marking the upper border of the deep cuff in the midline, 2 to 3 cm below the umbilicus (Figure 7). Catheter tip was placed in pelvis. In patients with fatty mesentery, redundant sigmoid and large greater omentum, catheter was fixed to anterior abdominal wall using a suture passer with 1-0 prolene sutures.
Peritoneal dialysis was started on the tenth day with a volume of 2000 mL. If there was no leakage, the PD volume was gradually increased to 8000 mL (4×2000 mL) over the coming 3-4 days. All patients were followed up and monitored for any early and late complications. Early post-insertion complications were defined as those that occurred during the procedure or within 30 days after insertion, and late complications were those that occurred after 30 days.
TABLE 1: Patient
characteristics.
|
S.No |
Age/ Sex |
ASA Grade |
Comorbidity |
Previous surgery |
Post op stay (days) |
|
1 |
76/ F |
4 |
DM, HTN, CAD |
AV Fistula (
Thrombosed) |
5 |
|
2 |
60/ F |
4 |
PTCA, ADPKD, HCV+ |
|
2 |
|
3 |
68/ F |
3 |
|
CAPD |
|
|
4 |
32/ F |
3 |
|
|
2 |
|
5 |
67/ F |
3 |
|
|
3 |
|
6 |
18/ M |
3 |
Downs, Seizure Disorder |
Ventral hernia |
|
|
7 |
50/ F |
4 |
Graft rejection |
Renal
Transplant Appendectomy, LC |
|
|
8 |
71/ M |
3 |
COPD |
|
2 |
|
9 |
61/ M |
4 |
CVA |
|
|
|
10 |
64/ M |
3 |
|
|
2 |
|
11 |
53/ F |
3 |
|
|
|
|
12 |
77/ M |
4 |
CVA, AF, HYPOTHYROID |
|
|
|
13 |
66/ M |
3 |
COPD |
|
|
|
14 |
77/ M |
4 |
CVA, AF, HYPOTHYROID |
|
1 |
|
15 |
66/ M |
4 |
COPD, Encephalopathy |
CAPD |
2 |
|
16 |
50/ F |
3 |
|
|
2 |
|
17 |
40/ M |
4 |
Pleural effusion |
|
2 |
|
18 |
70/ M |
3 |
Parkinson |
|
1 |
|
19 |
61// F |
3 |
|
|
2 |
|
20 |
71/ M |
3 |
Hypothyroid |
|
|
|
21 |
57/ F |
3 |
HCV |
|
2 |
|
22 |
68/ F |
3 |
|
|
2 |
|
23 |
65/ M |
3 |
|
|
2 |
|
24 |
55/ M |
3 |
COPD, HBV |
Decortication |
2 |
|
25 |
16/ F |
4 |
Hypothyroid, Spina Bifida, Seizure |
|
2 |
|
26 |
71/ M |
4 |
Hypothyroid, DVT |
|
4 |
|
27 |
62/ F |
3 |
|
|
2 |
|
28 |
85/ F |
3 |
POTT'S SPINE |
AV Fistula
(Failure) |
7 |
|
29 |
61/ M |
3 |
|
CAPD |
2 |
|
30 |
63/ F |
3 |
|
|
|
3. Results
Patient characteristics are summarized in (Table 1). There were 15 males and 15 females with a mean age of 61.9 years (range, 18-85 years). The etiology of ESRD included 21 patients (32.2%) with diabetes Mellitus, 1 patient had ADPDK. 28 patients had associated hypertension and 6 patients had concomitant CAD.
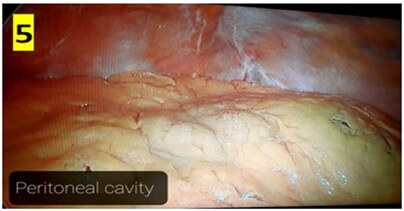

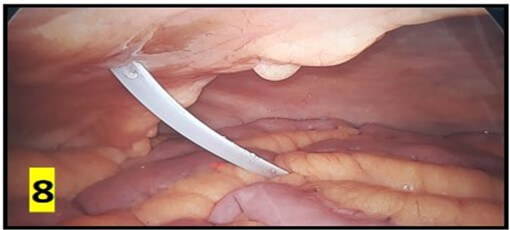
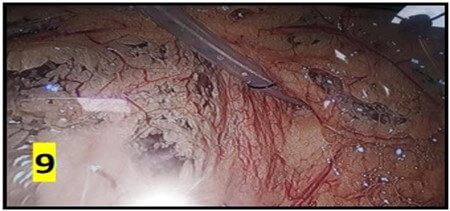
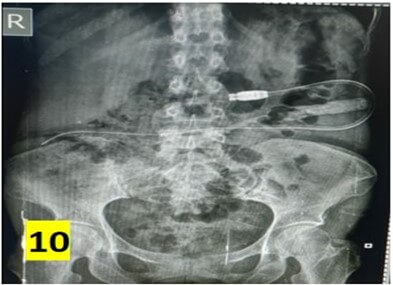
The average operating time was 40 min and ranged between 15-160 min. Post-insertion complications are summarized in (Table 2). No organ injury was observed in any patient. There were no instances of peri-catheter leakage or bleeding in post-operative period. Average hospital stay was 5 days. Outflow failure occurred in 6 (20%) patients. All these patients underwent X-ray abdomen which revealed catheter mal-position in 3 patients (Figure 10). 5 cases underwent a repeat diagnostic laparoscopy through the same 10 mm port site. 2 patients had omental wrapping (Figure 9) and 1 patient had catheter tip buried within bowel loops (Figure 8). All catheters were salvaged and catheter fixation was done in the midline. 1 patient had catheter blockade due to fibrin plug managed with flushing of catheter with normal saline.
TABLE 2: Complications.
|
Complication |
No. Of patients |
|
Dysfunction |
6 |
|
Wound infection |
0 |
|
Exit site infection |
0 |
|
Bleeding |
0 |
|
Wound leakage |
0 |
|
Malposition |
3 |
|
Blockade |
4 |
|
Peritonitis |
5 |
|
Mortality |
2 |
TABLE 3: Management of
complications.
|
S.No |
Age/Sex |
Complication |
Intervention |
Remarks |
Clavien Dindo |
|
1 |
76/F |
Peritonitis |
Antibiotics |
Death |
V |
|
2 |
68/F |
Malposition,
Peritonitis |
Reoperation/ Removal |
Removal |
IIIb |
|
3 |
61/M |
Peritonitis |
Antibiotics |
Salvaged |
II |
|
4 |
77/M |
Malposition/
Interbowel tip |
Reoperation |
Salvaged |
IIIb |
|
5 |
66/M |
Malposition/Omental
wrapping |
Reoperation |
Salvaged |
IIIb |
|
6 |
71/M |
Malposition |
Reoperation |
Salvaged |
IIIb |
|
7 |
71/M |
Peritonitis |
Antibiotics/ Removal |
Death |
V |
|
8 |
62/F |
Malposition/Omental
wraping |
Reoperation |
Salvaged |
IIIb |
|
9 |
61/F |
Peritonitis |
Antibiotics |
Salvaged |
II |
|
10 |
31/F |
Blockade |
Flush |
Salvaged |
I |
Peritonitis occurred in 5 (16%) patients. Peritonitis was defined as a turbid effluent with a leukocyte count >100 cells/μL, with more than 50% polymorphonuclear cells and a positive microbiological culture. The term “catheter infection” is used to indicate infection in the exit site, tunnel, or both. There was no incidence of catheter site infection in the case series. 3 patients were treated successfully with culture directed antibiotics, and 2 patients required removal of catheters. There were 2 deaths during the study period, one due to peritonitis and other due to underlying cardiac disease. There was no mortality related to surgical intervention in the study group.
4. Discussion
Globally CKD forms a critical public health issue owing to its high prevalence, morbidity and mortality. In developing countries non-communicable diseases like CKD have not received much attention owing to deficiency of resources and the high burden of communicable diseases [3]. In India, lack of disease specific database and registry and lack of sufficient dedicated centers of care & access to RRT, the true disease load of CKD is not known. Around 90% of CKD patients in need of RRT die because of lack of accessible care, and 60% of patients stop their treatment on financial grounds. India is predicted to have the world’s largest diabetic population by 2030. An estimated 50% of CKD patients are diagnosed when the eGFR is <15 ml/min per 1.73 m2 owing to challenges in health care access [4]. Such dismal figures highlight the need for robust screening programs for populations at risk for CKD. The reported prevalence of CKD ranges from less than 1% to 13%. The International Society of Nephrology’s Kidney Disease Data Center Study reported a prevalence of 17% [5].
The concept of dialysis was introduced by pioneers like Willem Kolff and Belding Scribner. This evolution led to several striking changes in the epidemiology, economics and ethical model for the treatment of kidney failure. In India, hemodialysis (HD) was introduced in 1962, kidney transplantation was initiated in 1971, and peritoneal dialysis (PD) began to gain importance in 1991.
HD is the most common treatment modality followed by transplantation, and PD is an extreme third. The presence of HD units in urban locations makes PD an attractive modality. However, for majority of patients in rural places, lack of health insurance coverage and expensive continuing costs (approximately 20,000 to 25,000 INR monthly) pose real hindrances for continuation of treatment. Despite these obstacles the utilization of PD is increasing and India is estimated to have over 8500 patients on PD. Timely supply of PD fluid to remote and poorly accessible areas and nonavailability of speciality hospital access for evaluation and treatment of PD-related complications are crucial challenges. The problems of un-sanitary living conditions, non-availability of separate clean rooms for PD and clean water for proper hand hygiene before PD are some of the challenges which need to be addressed. Like other developing country, India has many specific situations and challenges that preclude the early diagnosis and management of CKD. In-equitable distribution of health facilities and expert specialist care is a real time challenge.
Peritoneal Dialysis (PD) is widely used in the management of end-stage renal failure [6]. PD as a treatment for patients with end-stage renal disease (ESRD) provides a viable alternate option to hemodialysis (HD). In multiple studies, PD has shown increased two-year survival rate (20-48%) as compared to HD with a lower overall treatment related costs [7]. CAPD catheter insertion by the open method requires minimal gadgets and is a technically easier procedure. Its utility, however, is limited by a painful incision, wound related issues and an un-avoidable tube dislocation problem. Comparably, the laparoscopic alternative offers lesser in-hospital stay, lesser pain and wound related complications, a better patient quality, lesser readmissions and thereby a higher patient gratification [8]. PD is presently considered the desirable treatment for patients with congestive heart failure, vascular access failure and as a bridging therapy to kidney transplantation [9].
Since the advent of laparoscopy many placement techniques have been described. The techniques differ in aspects of technical simplicity, need for additional procedures, outcomes, complications and patient satisfaction. Additional procedures required during Lap PD placement comprise of adhesiolysis, catheter fixation, omentopexy / omentectomy, and rectus sheath tunneling [10, 11]. The Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) committee have recently recommended to appraise these different techniques. The Society encourages a laparoscopic approach with adhesiolysis, rectus sheath tunneling, and omentopexy as the gold standard technique. According to their guideline, this procedure offers the lowest rate of postoperative PD catheter dysfunction and should be the preferred placement technique in adults [12].
The most frequently reported complications of PD are peritonitis, catheter exit site infections, mechanical complications, and leakage of dialysate. The long-term functioning of peritoneal dialysis is ascertained by a functional peritoneal access. Complication-free access relies largely upon a precise catheter placement technique and stringent attention to intraoperative findings and anatomical details at the time of catheter placement [13]. The International Society for Peritoneal Dialysis (ISPD) recommends that each center catering to PD patients should have a specialized dedicated team for the care of PD patients with an experienced surgeon who analyzes the data regularly.
Ten percent of PD catheters may have primary non-function. Several factors may be responsible for non-function like catheter malposition or migration, intraluminal obstruction due to fibrin strands or blood clots and extraluminally by omental wrapping or adhesions. Primary non-function is one of the most leading causes of PD catheter removal. In an attempt to improve catheter functioning and survival few authors advocate performing omentectomy/omentopexy at the index operation in order to prevent omental wrapping and catheter occlusion. The decision to perform prophylactic omentectomy in peritoneal catheter placement remains at the discretion of the surgeon.
4.1. Catheter Malfunction
Catheter malfunction is defined as an inadequate inflow and/or outflow of dialysate fluid. Catheter inflow problems may merely be due to kinking of catheter or internal catheter obstruction [14]. Catheter malfunction has troubled PD patients since inception of PD in 1968, leading to frustration among doctors and patients likewise. No insertion technique has been infallible to prevent such complications.
4.2. Obstruction
Inflow and outflow failures may be due to multiple intra and extra-luminal causes. Blood or fibrin clot, distended colon due to constipation [8], fibrin sheath encapsulation, omental wrapping can result in outflow failure. Finally peritoneal cavity compartmentalization due to adhesions, or migration of the catheter tip outside the pelvis prevents adequate flow of dialysate fluid [15, 16, 17]. The omentum is a frequent source of catheter blockage. Omental wrapping leads to removal or exchange of PD catheters in 5-15% of cases [18]. Surgeons have formulated many strategies to reduce catheter obstruction and failure due to omental causes which include omentopexy, omentectomy and omental folding. Ogunc et al. extensively studied the role of omentopexy and concluded that the omental related complication rate is 0 % with routine use of this technique [19].
Nicholson first described omentectomy as an supportive procedure for open CAPD procedure leading to a significant increase in the catheter survival (P<0.01). Ladd et al. described a series of 163 pediatric patients, and concluded significantly reduced catheter failure rates (23% without omentectomy vs. 15% with omentectomy) with the performance of omentectomy [20]. Goh et al. found an 83% 1-year catheter survival rates in a series of 18 patients with an omental folding technique by performing plication of the omentum with silk sutures [21].
In our series omental wrapping was found in two cases, requiring re-positioning and catheter fixation using suture passer. We did not perform routine omentopexy, omentectomy or omental wrapping. We recommend to fix the catheter with the tip in the pelvis. The suture used for fixation is a mono filament non absorbable suture. The needle passer should ideally pass through the midline with two separate but close entry points and the fixation need not be very tight. Care should be taken if the median or medial umbilical ligaments are lax as they can additionally need addressal to prevent catheter blockage and malfunction.
The catheter material design and type remains an area of active research and hold immense potential for improvement. The search for an ideal tissue-phobic, hydro-phobic, bacterio-phobic, non reactive, cheap, non carcinogenic material for catheters should continue. Catheter design to maximise the holes for delivery of dialysate and to increase the area of catheter with the holes is also an active field for improvement. Cuffs designs that provide good sealing and plugging need to be worked upon.
4.3. Migration
Laparoscopic fixation prevents migration or malposition of catheter out of the pelvis. However there have been reported cases of increased risk for obstructive adhesions, internal hernias and infection [22]. In a series of 19 patients who underwent laparoscopic catheter fixation to the uterus or peritoneum showed reduced catheter failure rates. However, the series had higher leak rates because of the requirement for an excess laparoscopic port [8].
In our series, 3 patients had catheter dysfunction due to migration of catheter tip in early post operative period. One patient had additional omental wrapping of the catheter tip. All patients were managed with repeat laparoscopy through same 10 mm port with repositioning of catheter and suture fixation using a suture passer.
PD catheter fixation was done in cases with large omentum or redundant bowel loops leading to intraoperative outflow problems. Catheter fixation was done in selected cases on discretion of surgeon and nephrologist after assessment of intra-operative findings.
4.4. Hernias
There is an increased risk of abdominal wall hernias in patients on CAPD due to circulation of peritoneal dialysate leading to an increased intra-abdominal pressure and tension on the abdominal wall. In a series of 142 patients undergoing catheter placement by nephrologist with an open technique, Del Peso et al. found a hernia rate of 37%, primarily umbilical hernias [23]. Hernia rates in laparoscopic assisted PD catheter placement is not well described in literature. Schmidt et al. described incisional or port site hernias in 6.3% cases [24]. In order to prevent complications during dialysis, ISPD and SAGES both suggest surgical repair of abdominal wall [12, 25]. There was no hernia formation in our study group. Port site was closed in 2 layers in all patients using the Flip-Flap technique [26].
4.5. Leakage
Dialysate fluid leak is a common problem after PD catheter insertion and has been reported in 0 - 12.8 % of patients after laparoscopic insertion. It can be early (<30 days) or late (>30 days). Early leaks are usually from the catheter insertion site or surgical wounds which may be related to placement technique and/or the timing of commencement of CAPD after surgery.
The relative incidence of dialysate leakage has been recorded to be more in infants (up to 18 %) than in children and adults which seems possible due to their thinner abdominal walls [27]. Peritoneal dialysate leak can occur through surgical incisions and catheter entry points. In patients with dialysate leakage, dialysate infusion rate and volume might need to be lowered, though some patients may require complete cessation of the dialysis sessions. Leak rates can vary from 2% to 19% with basic laparoscopic techniques and can be lesser (0 to 5%) with advanced techniques [12].
Schmidt et al. (n=43) recorded a leak rate of 13% in their series [24]. Juergensen et al. described a higher leak rates because of the requirement for an excess laparoscopic port [8]. No leakage of dialysate fluid was observed either through entry site or port site in our study owing to use of single port in a non dependant area and meticulous closure of port sites in 2 layers. We also adopted a policy of delayed skin suture removal ( > 2 weeks ) for the port sites.
4.6. Visceral Injury
Injuries to the small or large bowel are uncommon after laparoscopic PD insertion due to the direct visualization of the catheter insertion into the abdomen. Bowel injury during laparoscopic placement may occur in patients requiring adhesiolysis but no incidents have been reported in the adult literature. Visceral injuries have been documented during blind percutaneous and open techniques. No abdominal visceral injury occurred in our case series.
4.7. Exit Site and Cuff Infection
The term “catheter infection” is used to indicate infection in the exit site, tunnel, or both. Infection of the skin at the catheter exit site or rarely the skin overlying the insertion site may be an early or late complication. Staphylococcus aureus is the most common cause of exit site and tunnel infections [28]. The initial treatment is usually oral antibiotics and gentamycin cream applied locally at exit site [29]. Wu et al. described 23 patients, with replacement of 26 catheters with entire sub-cutaneous tubing just above the internal cuff with no requirement of disruption in PD dialysis [30]. No exit site or tunnel infections were recorded in our study group. All patients received preoperative surgical prophylaxis: chlorhexidine bath, clipping, povidone iodine scrub and Inj Vancomycin 500mg iv before induction.
4.8. Peritonitis
The incidence of peritonitis after PD catheter insertion has been reported between 0 - 11 %. The management consists of intravenous and intra-peritoneal antibiotics based on culture results. Catheter removal may be necessary in refractory cases not responding to antibiotics and patients with fungal peritonitis. Peritonitis is the most common cause of cessation of continuation of CAPD [31]. Bacteria intrude the peritoneal cavity through catheters via the intra-luminal or peri-luminal routes. In our cohort, peritonitis occurred in 5 (16%) patient. In our study, 03 patients were treated successfully with culture directed antibiotics of effluent and continued on CAPD after cessation of infection, and 2 patients required removal of catheters. One patient died due to peritonitis related sepsis despite catheter removal.
5. Conclusion
Laparoscopic assisted PD placement is a safe and feasible procedure. Laparoscopy has an added advantage of placing catheter under vision, assessment and addressal of intra-abdominal conditions that can contribute to catheter malfunction. Requirement of additional procedures like fixation, omentectomy or omentoplasty can also be done on a need basis with addition of minimal morbidity. Our modified technique of single port laparoscopy avoids a large open scar, prevents leakage and has minimal wound related complications. There is no need for any extra ports and hence port site complications are reduced to a minimal.
We recommend the industry works in a close tandem with the nephrologists and surgeons for optimal design of catheters with long working lives. Catheter material and design should be individualised as per body habitus and intra operative requirements.
Conflicts of Interest
None.
Abbreviations
CAPD: Continuous ambulatory peritoneal dialysis
ESRD: End-stage renal disease
PD: Peritoneal dialysis
REFERENCES
[1] G H Poole, P Tervit “Laparoscopic
Tenckhoff catheter insertion: A prospective study of a new technique.” Aust N Z J Surg,
vol. 70, no. 5, pp. 371-373, 2000. View at: Publisher Site | PubMed
[2] Aakash H Gajjar, Diane H Rhoden,
Pranay Kathuria, “Peritoneal dialysis catheters: laparoscopic versus
traditional placement techniques and outcomes.” Am J Surg, vol. 194, no.
6, pp. 872-875, 2007. View at: Publisher Site | PubMed
[3] Georgi
Abraham, Santosh Varughese, Thiagarajan Thandavan, et al. “Chronic kidney
disease hot spots in developing countries in South Asia.” Clin Kidney J,
vol. 9, no. 1, pp. 135-141, 2016. View at: Publisher
Site | PubMed
[4] Santosh
Varughese, G T John, S Alexander, et al. “Pre-tertiary hospital care of
patients with chronic kidney disease in India.” Indian J Med Res, vol.
126, no. 1, pp. 28-33, 2007. View at: PubMed
[5] Bogdan
Ene-Iordache, Norberto Perico, Boris Bikbov, et al. “Chronic kidney disease and
cardiovascular risk in six regions of world (ISN-KDDC): A cross sectional
study.” Lancet Glob Health, vol. 4, no. 5, pp. e307-e319, 2016. View at: Publisher Site | PubMed
[6] Melanie Wyld, Rachael Lisa Morton,
Andrew Hayen, et al. “A systematic review and meta-analysis of utility-based
quality of life in chronic kidney disease treatments.” PLoS Med, vol. 9,
no. 9, pp. e1001307, 2012.
View at: Publisher
Site | PubMed
[7] Vikas Makkar, Manish Kumar, Rajesh
Mahajan, et al. “Comparison of outcomes and quality of life between
hemodialysis and peritoneal dialysis patients in indian ESRD population.” J Clin Diagn Res,
vol. 9, no. 3, pp. 28-31, 2015. View at: Publisher Site | PubMed
[8] Erika Juergensen, Diane Wuerth, Susan
H Finkelstein, et al. “Hemodialysis and peritoneal dialysis: Patients’
assessment of their satisfaction with therapy and the impact of the therapy on
their lives.” Clin J Am Soc Nephrol, vol. 1, no. 6, pp. 1191-1196, 2006.
View at: Publisher Site | PubMed
[9] A. Shetty, D.G. Oreopoulos
“Peritoneal dialysis: Its indications and contraindications.” Dial
Transplant, vol. 29, no. 2, pp. 71-77, 2000.
[10]
Aakash
H Gajjar, Diane H Rhoden, Pranay Kathuria, et al. “Peritoneal dialysis
catheters: Laparoscopic versus traditional placement techniques and outcomes.” Am
J Surg, vol. 194, no. 6, pp. 872- 876, 2007. View at: Publisher Site | PubMed
[11]
D
I Watson, D Paterson, K Bannister “Secure placement of peritoneal dialysis
catheters using a laparoscopic technique.” Surg Laparosc Endosc, vol. 6,
no. 1, pp. 35-37, 1996. View at: PubMed
[12]
Stephen
Haggerty, Scott Roth, Danielle Walsh, et al. “Guidelines for laparoscopic
peritoneal dialysis access surgery.” Surg Endosc, vol. 28, no. 11, pp.
3016- 3045, 2014. View at: Publisher
Site | PubMed
[13]
Shyh-Chuan
Jwo, Kuo-Su Chen, Chin-Chan Lee, et al. “Prospective randomized study for comparison
of open surgery with laparoscopic-assisted placement of tenckhoff peritoneal
dialysis catheter: A single centre experience and literature review.” J Surg
Res, vol. 159, no. 1, pp. 489-496, 2010. View at: Publisher Site | PubMed
[14]
C
P Cacho, M J Tessman, L N Newman, et al. “Inflow obstruction due to kinking of
coiled catheters during placement. Perit Dial Int, vol. 15, no. 6, pp.
276-278, 1995. View at: PubMed
[15]
Nasim
Shahbazi, Brendan B McCormick “Peritoneal dialysis catheter insertion strategies and
maintenance of catheter function.” Semin Nephrol, vol. 31, no. 2, pp. 138-151, 2011. View
at: Publisher
Site | PubMed
[16]
Tuncay Yilmazlar, Turkay Kirdak, Serpil
Bilgin, et al. “Laparoscopic findings of peritoneal dialysis catheter malfunction and
management outcomes.” Perit Dial Int, vol. 26, no. 3, pp. 374-379, 2006. View
at: PubMed
[17]
J A Diaz-Buxo “Management of peritoneal catheter
malfunction.” Perit Dial Int, vol. 18, no. 3, pp. 256-259. View at: PubMed
[18]
M.
Kaya, M. E. Boleken, M. Soran, et al. “Laparoscopic omental folding: A new
procedure to prevent omental wraps of continuous peritoneal dialysis catheters.”
Eur Surg, vol. 44, no. 5, pp. 345-348, 2012. View at: Publisher Site
[19]
G
Oğünç, M Tuncer, D Oğünç, et al. “Laparoscopic omental fixation technique versus open
surgical placement of peritoneal dialysis catheters.” Surg Endosc, vol.
17, no. 11, pp. 1749-1755, 2003. View at: Publisher Site | PubMed
[20]
Alan
P Ladd, Francine D Breckler, Nathan M Novotny “Impact of primary omentectomy on
longevity of peritoneal dialysis catheters in children.” Am J Surg,
vol. 201, no. 3, pp. 401-404, 2011. View
at: Publisher
Site | PubMed
[21]
Y
Heng Goh “Omental folding: A novel laparoscopic technique for salvaging
peritoneal dialysis catheters.” Perit Dial Int, vol. 28, no. 6, pp.
626-631, 2008. View at: PubMed
[22]
Jodie
H Frost, Atul Bagul “A brief recap of tips and surgical manoeuvres to enhance
optimal outcome of surgically placed peritoneal dialysis catheters.” Int J
Nephrol, vol. 2012, pp. 251584, 2012. View at: Publisher Site | PubMed
[23]
Gloria
Del Peso, María Auxiliadora Bajo, Olga Costero, et al. “Risk factors for
abdominal wall complications in peritoneal dialysis patients.” Perit Dial
Int, vol. 23, no. 3, pp. 249-254, 2003. View at: PubMed
[24]
Sven
C Schmidt, Cosima Pohle, Jan M Langrehr, et al. “Laparoscopic-assisted
placement of peritoneal dialysis catheters: Implantation technique and
results.” J Laparoendosc Adv Surg Tech A, vol. 17, no. 5, pp. 596-599,
2007. View at: Publisher
Site | PubMed
[25]
Ana
Figueiredo, Bak-Leong Goh, Sarah Jenkins, et al. “Clinical practice guidelines
for peritoneal access.” Per Dial Int, vol. 30, no. 4, pp. 424-429, 2010.
View at: Publisher
Site | PubMed
[26] Ajaz
Ahmed Wani, Suhail Khuroo 1, Saurabh Kumar Jain, et al. “The “Flip-Flap”
technique for laparoscopic port-site closure - Description of a novel,
cost-effective technique with review of literature.” Surg J (N Y), vol.
7, no. 3, pp. e161-e171, 2021. View at: Publisher
Site | PubMed
[27] Jennifer Phan, Steve Stanford, Joshua
J Zaritsky, et al. “Risk factors for morbidity and mortality in pediatric
patients with peritoneal dialysis catheters.” J Pediatr Surg, vol. 48,
no. 1, pp. 197-202, 2013. View at: Publisher Site | PubMed
[28] Georgi Abraham, Evgeije Savin,
Anthony Ayiomamitis, et al. “Natural history of exit-site infection (ESI) in patients on continuous
ambulatory peritoneal dialysis (CAPD),” Peritoneal Dialysis International:
Journal of the International Society for Peritoneal Dialysis, vol. 8, no. 3, pp. 211-216, 1988. View at: Publisher Site
[29]
Jonny,
Rudi Supriyadi, Rully Roesli, et al. “A Simple Tenckhoff Catheter Placement
Technique for Continuous Ambulatory Peritoneal Dialysis (CAPD) Using the
Bandung Method.” Int J Nephrol, vol. 2020, pp. 4547036, 2020. View at: Publisher Site | PubMed
[30] Y M Wu, M K Tsai, S H Chao,et al. “Surgical management of refractory exit-site/tunnel infection of Tenckhoff catheter: technical innovations of partial replantation.” Perit Dial Int, vol. 19, no. 5, pp. 451-454, 1999. View at: PubMed
[31] L. Solaro “Infectious complications of continuous ambulatory peritoneal dialysis.” G Batteriol Virol Immunol, vol. 79, no. 7-12, pp. 244-249, 1986. View at: PubMed
