Received: Tue 19, Dec 2023
Accepted: Thu 18, Jan 2024
Abstract
Objective: Oral cancers are one of the most common cancers in India. Tongue and lower gingiva-buccal sulcus are the most common subsites in oral cancers. Good reconstruction after resection of primary is a demanding task and determines quality of life. This study reviews our experience with infrahyoid flap (IHF) in reconstruction of small and medium sized defects after oral cancer resection. Materials and Methods: This study retrospectively analyzes all patients of oral cavity squamous cell carcinoma who underwent IHF flap by a single surgeon from July 2018 till June 2022 with a median follow-up of 12 months. Results were analyzed based on characteristics of the patients, site and size of defect, postoperative complications and clinical outcome. Results: Out of 14 patients, 4 patients had skin paddle necrosis (2 total and 2 partial). None of the patient suffered a total flap loss. All cases of partial flap loss resolved with conservative treatment and none of the patient required a revision surgery or developed orocutaneous fistula. The largest flap size was 9 × 4.5 cm. Conclusion: The infrahyoid myocutaneous flap is a fairly reliable and easy to perform flap for small and medium‑sized defects of the oral cavity.
Keywords
Head and neck cancer, infrahyoid flap, oral cancer, pedicled flap, reconstruction
1. Introduction
According to Globocan 2020, oral cancers amount to 10.3% of total cancer burden in India. A cosmetically and functionally good reconstruction is a major challenge in treatment of such cancers. Free flaps are gaining the priority over various traditional work horse flaps for reconstruction. But are associated with disadvantages like prolonged operating time, need of microvascular set up and expertise, prolonged hospitalization, chances of revision/exploration surgeries, donor site morbidities etc. In the era of free flaps, pedicled flaps are often overlooked, which are reliable, easy to perform and have consistent anatomy.
Introduced first by Wang and Shen in 1980, infrahyoid myocutaneous flap (IHF) is a versatile and reliable flap used in reconstruction for small- and medium-sized defects following resection of head and neck cancers. The main advantages of infrahyoid flap include its low surgical complexity and ease of harvest. Also, when compared to free flap, infra hyoid flap has shorter operating time, shorter hospital stay, low cost, lesser post operative complication and lesser donor site morbidity. Other added advantages are, that infrahyoid flap is relatively hair less, can be easily raised along with neck dissection and can be easily incorporated into the oral cavity from the same neck incision. The myocuteneous paddle is outside of lymphatic drainage area, hence risk of nodal recurrence is absent. Like any other flap complications of IHF is mainly because of compromised venous drainage, which may range from 3% to 47% [1].
However various contraindications of IHF flap include previous thyroid surgery, neck dissection, and presence of N3 neck metastasis, high face defects, and large sized defects [2-4]. Here, we present our experience of management of oral cancer with IHF reconstruction at tertiary care center.
1.1. Anatomy of Infra Hyoid Flap
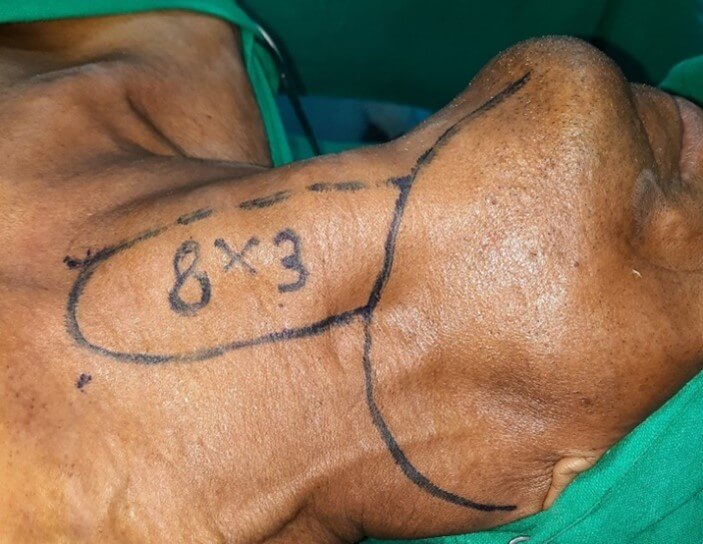
Infrahyoid flap is composite flap consisting of skin and subcutaneous tissue, platysma and underlying muscles, which include sternohyoid, sternothyroid and superior belly of omohyoid. Vascular basis of this flap is superior thyroid artery, which is a branch of external carotid artery. Venous drainage occur via anterior jugular vein and superior thyroid vein. Flap can be designed either vertically or horizontally oriented. Medial border of the flap corresponds to midline of the neck, from thyroid cartilage to sternal notch. Flap can be extended laterally as much as possible to allow primary closure of the donor site. Maximum dimension of the harvested flap is up to 10-12 cm × 6-8 cm. (Figure 1) [5, 6].
2. Material and Methods
The study was conducted in surgical oncology department of our institute. Patients with squamous cell carcinoma of oral cavity, clinically T1-T3 and N0-N1 stage, with anticipated defects of small to medium defects were included for the study. However, patients with larger tumor size, advance nodal stage, recurrent tumor, prior radiation, prior neck dissection and prior thyroid surgery were excluded from the study. Internal review board authorization was approved for this study. The study was done from July 2018 to June 2022, including follow up of 1 year. Total 14 patients were included in the study. All patients were thoroughly evaluated and planned for surgery after informed consent. All patients had squamous cell carcinoma of oral cavity involving different subsites. Patients were operated by a single surgeon, using the same technique.
2.1. Surgical Technique
The design of the flap was marked incorporating the incision for neck dissection. Upper limit of the flap was kept at the level of hyoid bone, the lower limit at the suprasternal notch, medial limit at the midline. Lateral limit was decided based on size of defect and feasibility to close the donor site primarily. Flap harvest was carried out after completion of lymph node dissection. Special care was taken during neck dissection to preserve the superior thyroid pedicle at its origin from internal jugular vein and external carotid artery. Skin incision is taken along the marked dimensions of the flap. Anterior jugular vein is identified distally and included in the flap. Sternohyoid and sternothyroid muscles were divided at the suprasternal notch and included in the flap. Superior belly of omohyoid was divided at level of IJV and also included in the flap. Flap was then progressively raised cranially in the avascular plan over thyroid capsule till the upper pole of the gland was reached.
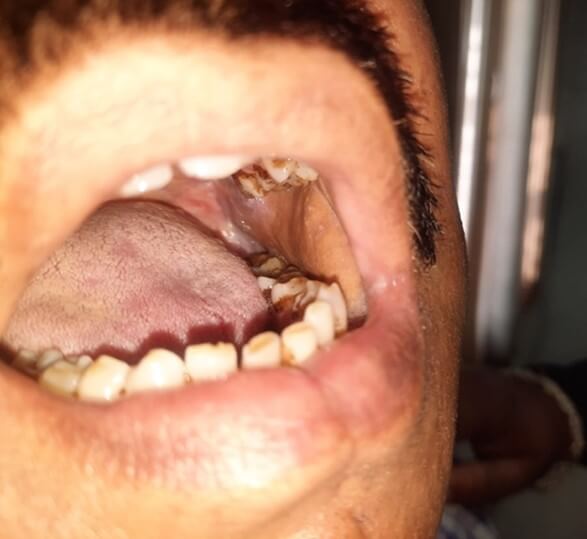
Here the superior thyroid artery was identified with all its branches. Multiple tiny branches from superior thyroid artery entering infrahyoid muscle were carefully preserved. Posterior branches supplying thyroid artery were cut and ligated, and the main trunk of artery was taken along with the flap. The sternothyroid muscle was detached from the thyroid cartilage and cricothyroid artery was cut and ligated at midline of the neck and included with the flap. External branch of the superior laryngeal nerve was carefully preserved in all cases during this step. Once the flap was made freely mobile after detaching hyoid insertion of sternohyoid muscle, it was transferred to the defect in the oral cavity (Figure 2). Neck drain was kept, and all patients were extubated after completion of surgery. Patients were managed post-operatively with antibiotics, analgesics and other symptomatic drugs. After recovery patients were discharged and followed up in outpatient department till 1 year of duration (Figure 3).

3. Results
Total 14 patients were included in the study. In all patients tumor excision, neck dissection and infra hyoid flap was done. Male female ratio was 11:3. Age of patients ranged between 45-60 years. T stage of tumor in all patients was T2 to T3, whereas N stage of tumor in all patients was N0 to N1. Largest defect size after excision of primary tumor was 11 × 6 cm. Duration for the surgery was 3-4 hours. None of the patient suffered major complications in post-operative period, and average hospital stay was 4-7 days. In all patient’s donor site healed well, with soft and supple scar. None of the patients suffered donor site morbidity (Figure 4).
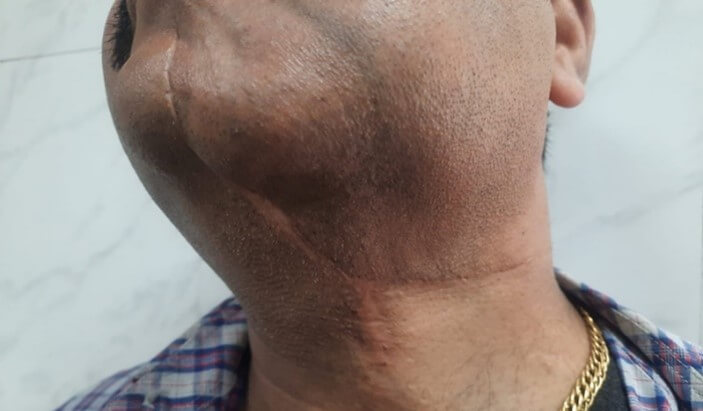
None of the patients underwent revision surgery and none of the patient had total flap loss. 4 patients had minor flap complications, out of which three patients had partial, superficial skin necrosis of the flap which was managed conservatively and epithelized later. Only one patient had complete but epidermal necrosis of skin of flap, which eventually healed by conservative measures. None of the patient developed major complications, orocutaneous fistula or radiation necrosis. None of the patient developed recurrence after one year of surgery. Table 1 showing patient’s demographic profile, characteristics of tumor and flap.
TABLE
1: Showing patient’s
demographic profile, characteristics of tumor and flap.
|
S No |
Age/sex |
Tumor characteristics |
Defect parameters |
Surgery |
Flap (cm) |
Duration of surgery (Hr) |
Adjuvant therapy |
Flap complication |
Repeat surgery |
Donor site morbidity |
Recurrence |
|
1 |
60/M |
Tongue, T2N1M0 |
6 × 4 cm, Left lateral tongue |
WLE+MRND |
7 × 3.5 myocutaneous |
3 |
radiation |
no |
no |
no |
no |
|
2 |
47/M |
Tongue, T2N1M0 |
5 × 3 cm, Left tongue ventral aspect |
WLE+MRND |
6 × 3 myocutaneous |
3 |
radiation |
Partial skin loss |
no |
no |
no |
|
3 |
53/M |
Lower alveolus T2N0M0 |
5 × 3 cm, Left lower alveolus |
WLE withMarginal mandibulectomy+SOND |
6 × 3.5 myocuteneous |
3.5 |
no |
no |
no |
no |
no |
|
4 |
55/F |
Tongue, T1N1M0 |
4 × 3 cm, Left lateral tongue |
WLE+MRND |
6 × 3.5
myocutaneous |
3 |
radiation |
Partial skin loss |
no |
no |
no |
|
5 |
60/F |
Tongue, T2N1M0 |
6 × 4 cm, right lateral tongue |
WLE+MRND |
8 × 3.5 myocutaneous |
3.5 |
radiation |
no |
no |
no |
no |
|
6 |
45/M |
Lower GBS T2N0M0 |
6 × 4cm, right lower alveolus, buccal mucosa |
WLE with Marginal mandibulectomy SOND |
7 × 4 myocuteneous |
3.5 |
radiation |
no |
no |
no |
no |
|
7 |
47/M |
Lower GBS T1N0M0 |
5 × 3cm, Left lower alveolus |
WE with Marginal mandibulectomy+ SOND |
6 × 3.5 myocuteneous |
3 |
no |
no |
no |
no |
no |
|
8 |
51/M |
Lower gingivobuccal sulcus T2N1M0 |
7 × 4cm, right lower alveolus, buccal mucosa |
WE with Marginal mandibulectomy+ MRND |
9 × 4.5 myocuteneous |
3.5 |
radiation |
no |
no |
no |
no |
|
9 |
53/F |
Tongue, T2N1M0 |
11 × 6 cm, right anterior tongue |
WLE+MRND |
8 × 4.5 myocutaneous |
3 |
radiation |
Partial skin loss |
no |
no |
no |
|
10 |
59/M |
Lower GBS T2N1M0 |
7 × 3.5cm, Left lower alveolus |
WE with Marginal mandibulectomy+ MRND |
8 × 3.5myocuteneous |
4 |
radiation |
no |
no |
no |
no |
|
11 |
54/M |
Lower GBS T2N0M0 |
6 × 3cm, Left lower alveolus |
WE with Marginal mandibulectomy+ SOND |
7 × 3.5 myocuteneous |
3.5 |
no |
no |
no |
no |
no |
|
12 |
60/M |
Tongue, T2N1M0 |
5 × 3 cm, Left lateral tongue |
WLE+MRND |
7 × 3.5
myocutaneous |
3 |
radiation |
no |
no |
no |
no |
|
13 |
56/M |
Tongue, T2N1M0 |
6 × 4 cm, Left lateral tongue |
WLE+MRND |
7 × 4 myocutaneous |
3 |
radiation |
Full
Epidermal loss |
no |
no |
no |
|
14 |
50/M |
Tongue T2N1M0 |
8 × 4 cm, Left lateral tongue |
WLE+MRND |
9 × 3.5
myocutaneous |
3 |
radiation |
no |
no |
no |
no |
4. Discussion
Head and neck reconstruction is always one of the most challenging reconstructions, because of cosmetic as well as functional needs. Choice of reconstructive procedure depends on location of defect, size of defect, components of defect, potential physiological implications, availability of resources and patient’s fitness/affordability for advanced procedures. Traditionally pectoralis major myocutaneous flap (PMMC) is non microsurgical work horse pedicled flap for head and neck reconstruction. However disadvantages include poor cosmesis, donor site morbidity, compromised oral competence, and complications related to gravitational pull. Also PMMC flap is often found to be bulky and cumbersome for small to medium defects [7, 8].
Recently, free flap is preferred at various centers. Free fibula flap is choice of reconstruction for bony defects, however free antero-lateral thigh and free radial forearm flaps are choice for soft tissue defects. Various limitations associated with free flap surgery include prolonged operative time, prolonged and monitored post-operative care, prolonged hospital stay, donor site morbidity, high cost and availability of microvascular set up etc. hence it may not be possible for centers having heavy patient load and limited resources [9-11]. Apart from work horse flaps, there are several flaps which have been used from time to time and are best suited for small to medium sized defects. Submental flap, nasolabial flap, supraclavicular flap and infrahyoid flap are ideal for small to medium defects of head and neck cancer.
Quality of life following oral cancer surgeries depends on choice of reconstruction used. Points of consideration include minimum to nil donor site morbidity, cosmetic appearance, inclusion of hair free skin in the oral cavity, ability to maintain oral competence, adequate flap bulk to withstand radiation necrosis. Clarimont et al. published the first case report of infrahyoid flap in 1977 for small medium sized defect and Wang et al. published the first case series in 1986 [12, 13]. Length of IHF pedicle and hence its reach depends on the level of bifurcation of carotid artery. The pedicle length based on superior thyroid artery ranges from 3-4 cm. Hence, in view of relatively short pedicle length, IHF is ideally suited for defects in the lower part of the oral cavity [14].
The advantage of IHF includes simultaneous neck dissection through the same incision, less time consuming, and gives aesthetically good results at the defect site. The main disadvantage of the flap is it is not suitable for higher face defects due to shorter pedicle length [15, 16]. Flap can be modified into sensate flap by preserving the nerve supply, which is derived by ansa cervicalis. This technique has twofold benefits, the flap is converted in to sensate flap and also underlying muscles are able to withstand ischemic changes after radiotherapy [17]. Venous congestion is the most common complication of this flap in post-operative period. Major venous drainage of infrahyoid area is through anterior and external jugular system and minor drainage is through the perforators of superior thyroid vessel. Hence preservation of venous drainage, avoidance of undue stretching over the pedicle, maintenance of flap laxity and post-operative positioning of the patients, are very important to avoid flap failure [18, 19].
When IHF is harvested, drainage is only via the perforator system. Minor shearing or pressure during harvest could easily damage the perforators, making the flap susceptible to skin necrosis. In their 1986 series including 112 patients Wang et al. reported no total flap losses; only partial skin necrosis in 11 patients. In our study also none of the patient had total flap necrosis [13]. The results of our study showed that IHF is quite reliable for reconstruction post oral cancer resection. Most flap losses were only partial skin necrosis. Only one flaps suffered complete skin necrosis. In all cases the defect healed by secondary intention and second surgical intervention was not required in any of the patients. None of the patients had a complete flap loss. None of the patients suffered donor site complication.
Some studies mention modified radical neck dissection (MRND) a contraindication for IHF and some studies recommend only SOND if IHF is planned. Gangloff et al. did not consider MRND a contraindication and in our series also, majority of patients underwent MRND without compromising the blood supply of flap [20-22].
5. Conclusion
The infrahyoid myocutaneous flap is a fairly reliable and easy to perform flap for small and medium‑sized defects of the oral cavity, without requiring additional incisions and donor site reconstruction. Although skin flap loss is concern, almost all complications resolve with conservative treatments.
Conflicts of Interest
None.
Funding
None.
Acknowledgements
Authors sincerely acknowledge the work of Mr Zia-Ul for efficiently assisting in the surgery.
Author Contributions
Idea, surgical procedure and manuscript writing: Vikas Sharma. Manuscript formatting: Sandhya Pandey. Patient management: Rastogi Madhup, Gandhi Ajeet, Sethi Rohini and Nidhi Anand.
REFERENCES
[1] HS Wang, JW Shen “Preliminary report
on a new approach to the reconstruction of the tongue.” Acta Acad Med Prim
Shanghai, vol. 7, pp. 256‑259, 1980.
[2] J Magrin, L P Kowalski, G E Santo, et
al. “Infrahyoid myocutaneous flap in head and neck reconstruction.” Head
Neck, vol. 15, no. 6, pp. 522‑525, 1993. View at: Publisher Site | PubMed
[3] Y F Zhao, W F Zhang, J H Zhao
“Reconstruction of intraoral defects after cancer surgery using cervical
pedicle flaps.” J Oral Maxillofac Surg, vol. 59, no. 10, pp. 1142‑1146, 2001. View at: Publisher
Site | PubMed
[4] Alberto Deganello, C René Leemans
“The infrahyoid flap: A comprehensive review of an often overlooked
reconstructive method.” Oral Oncol, vol. 50, no. 8, pp. 704‑710, 2014. View at: Publisher
Site | PubMed
[5] I
Eliachar, HZ Joachims, A Marcovich, et al. “Reconstruction of the larynx and
trachea with hyoid transposition Plastic and Reconstructive Surgery of the Head
And Neck.” New York Grune & Stratton pp. 259-265, 1981.
[6] I Eliachar, A Marcovich, Y Har Shai, et al.
“Arterial blood supply to the infrahyoid muscle, an anatomical study.” Head
Neck Surg, vol. 7, no. 1, pp. 8-14, 1984. View at: Publisher Site | PubMed
[7] Kyung S Koh, Jin Sup Eom, Insoo Kirk,
et al. “Pectoralis major musculocutaneous flap in oropharyngeal reconstruction:
revisited.” Plast Reconstr Surg, pp. 118, no. 5, pp. 1145-1149, 2006.
View at: Publisher
Site | PubMed
[8] Astrid L Kruse, Heinz T Luebbers,
Joachim A Obwegeser, et al. “Evaluation of the pectoralis major flap for
reconstructive head and neck surgery.” Head Neck Oncol, vol. 3, pp. 12,
2011. View at: Publisher
Site | PubMed
[9] Hung-Tao Hsiao, Yi-Shing Leu, Chang-Ching Lin
“Tongue reconstruction with free radial forearm flap after hemiglossectomy: a
functional assessment.” J Reconstr Microsurg, vol. 19, no. 3, pp.
137-142, 2003. View at: Publisher
Site | PubMed
[10] F C Wei, C S Seah, Y C Tsai, et al. “Fibula
osteoseptocutaneous flap for reconstruction of composite mandibular defects.” Plast
Reconstr Surg, vol. 93, no. 2, pp. 294-304, 1994. View at: PubMed
[11] Peirong Yu, Geoffrey L Robb
“Pharyngoesophageal reconstruction with the anterolateral thigh flap: a
clinical and functional outcomes study.” Plast Reconstr Surg, vol. 116,
no. 7, pp. 1845-1855, 2005. View at: Publisher
Site | PubMed
[12] A A Clairmont, J J Conley “Surgical technique
- The strap muscle flap.” J Otolaryngol, vol. 6, no. 3, pp. 200‑202, 1977. View at: PubMed
[13] H S Wang, J W Shen, D B Ma, et al.
“The infrahyoid myocutaneous flap for reconstruction after resection of head
and neck cancer.” Cancer, vol. 57, no. 3, pp. 663‑668, 1986. View at: Publisher
Site | PubMed
[14] V Laxmi, K Kaur, P Chhabra “Superior
thyroid artery: its origin, length, relations and branches.” Int Ann Med,
vol. 1, no. 4, 2017.
[15] R Lockhart, P Menard, P Chout, et al.
“Infrahyoid myocutaneous flap in reconstructive maxillofacial cancer and trauma
surgery.” Int J Oral Maxillofac Surg, vol. 27, no. 1, pp. 40‑44. View at: Publisher
Site | PubMed
[16] S Rojananin, N Suphaphongs, A J
Ballantyne “The infrahyoid musculocutaneous flap in head and neck
reconstruction.” Am J Surg, vol. 162, no. 4, pp. 400‑403, 1991. View at: Publisher
Site | PubMed
[17] H Wang “10 years experience on the infrahyoid
myocutaneous flap.” Zhonghua Er Bi Yan Hou Ke Za Zhi, vol. 26, no. 6,
pp. 332-4, 382, 1991. View
at: PubMed
[18] E A Meguid, A E Agawany “An
anatomical study of the arterial and nerve supply of the infrahyoid muscles.” Folia
Morphol (Warsz), vol. 68, no. 4, pp. 233-243, 2009. View at: PubMed
[19] Dian Ouyang, Xuan Su, Wei-Chao Chen,
et al. “Anatomical study and modified incision of the infrahyoid myocutaneous
flap.” Eur Arch Otorhinolaryngol, vol. 270, no. 2, pp. 675-680, 2013.
View at: Publisher
Site | PubMed
[20] J Bier, D Schlums, H Metelmann, et
al. “A comparison of radical and conservative neck dissection.” Int J Oral
Maxillofac Surg, vol. 22, no. 2, pp. 102‑107, 1993. View at: Publisher Site | PubMed
[21] Marc Moncrieff, Jessica Sandilla, Jonathan Clark, et al. “Outcome of primary surgical treatment of T1 and T2 carcinomas of the oropharynx.” Laryngoscope, vol. 119,no. 2, pp. 307‑311, 2009. View at: Publisher Site | PubMed
[22] P Gangloff, A Deganello, M L Lacave, et al. “Use of the infrahyoid musculocutaneous flap in soft palate reconstruction.” Eur J Surg Oncol, vol. 32, no. 10, pp. 1165‑1169, 2006. View at: Publisher Site | PubMed
