Received: Wed 19, Jul 2023
Accepted: Sat 29, Jul 2023
Abstract
Objective: The aim of this retrospective single-center study was to assess the effectiveness and safety of Rotarex thrombectomy and angioplasty (balloon dilatation and stenting) in the treatment of aortic endograft occlusion (AEO) after the endovascular repair of abdominal aortic aneurysm (AAA). Methods: Among a total of 683 patients who underwent the endovascular aneurysm repair (EVAR) for AAA between May 2010 and March 2020, 46 (6.7%) patients developed occlusions of the aortic endograft; 16 patients were treated with fogarty embolectomy, 4 were treated with AngioJet aspiration, and 26 patients with mechanical thrombectomy (Rotarex system). The average time for AEO was 5 weeks (range 2-11 weeks). Immediately after angiography, Rotarex thrombectomy was used to remove the thrombus and embolus. After thrombectomy, balloon dilation was performed, followed by stent-graft deployment. The patients’ characteristics, treatment details, and outcomes were collected and analyzed. Results: There were 26 patients with AEO, 20 males (76.9%), and with the mean age of 67 ± 12 years (51-87). The primary reasons for AEO were kinking or extrinsic compression of the graft limb and thrombosis. The circulation was successfully restored in all patients by mechanical thrombectomy. Additional dilation/stents were required for 24 patients (93.3%), and they were placed in the graft limbs, thus re-establishing patency. The complications were observed in five patients and included peripheral embolization blue toe syndrome found in three patients and ischemia-reperfusion injury found in two patients. No mortality or recurrent thrombosis was observed in the follow-up period, which was extended for 12 months. Conclusions: The combination of Rotarex thrombectomy and angioplasty is a safe and effective treatment method for AEO after endovascular AAA repair.
Keywords
Aortic endograft occlusion, Rotarex thrombectomy, angioplasty, limb ischemia, abdominal aortic aneurysm, endovascular aneurysm repair
1. Introduction
Aortic endograft occlusion (AEO) is a common critical complication related to endovascular aneurysm repair (EVAR) of abdominal aortic aneurysms (AAA) [1 ,2]. Culprit thrombus, which is assumed to be a primary cause of AEO, is generally caused by kinking of the stent-graft or tortuosity of the iliac artery [3]. Currently, several therapeutic strategies, such as fogarty embolectomy, bypass, pharmacologic thrombolysis, and thrombectomy, are used for the treatment of AEO [4-6]. Fogarty embolectomy has proven to be very successful in the treatment of thrombosed graft limbs. Nevertheless, it increases the morbidity and mortality associated with general anesthesia, surgical procedure, and damage of the endograft. An extra-anatomical bypass procedure, such as a femoro-femoral bypass graft, can be used as an alternative option. However, this procedure is debatable, given the relatively low number of prolonged patency. Moreover, a femoral artery cut down extends the duration of both hospitalization and time to ambulation [7].
Thrombolysis, similar to catheter-directed thrombosis, is a feasible treatment method that is widely accepted as an alternative to surgical intervention for AEO [8, 9]. Yet, pharmacological thrombolysis is often associated with extended periods of sufficient clot clearance. Thus, intensive care with careful hematological monitoring must be prolonged. In addition, hemorrhagic complications with prolonged infusions tend to occur commonly. Furthermore, contraindications to thrombolytic therapy are observed in approximately 20% of patients. A thrombectomy procedure can be regarded as a feasible and efficient way to treat patients with acute or subacute artery occlusion [10, 11]. The AngioJet system is a rheolytic thrombectomy device successfully used for the removal of thrombus from acute deep venous thrombosis. Nonetheless, there is a possibility for the occurrence of minor bleeding and access site hematoma [12]. Distal embolic sequelae present identified hazards associated with arterial thrombosis.
Practitioners have increasingly preferred a Rotarex device that combines thrombus fragmentation and suction [13, 14]. Using this approach has minimized the potential risks associated with peripheral embolization and thrombosis [15]. However, data regarding the effectiveness and safety of mechanical thrombectomy with Rotarex in AEO are scarce. Therefore, this retrospective single-center study aimed to evaluate the effectiveness and safety of Rotarex thrombectomy and angioplasty (balloon dilatation and stenting) in the treatment of AEO after AAA repair.
2. Methods
2.1. Patient Characteristics
Among a total of 683 patients who underwent the EVAR for AAA between May 2010 and March 2020, 46 (6.7%) patients developed occlusions of the aortic endograft. The average time of the occlusion development was approximately 5 weeks (ranging from 2-11 weeks). Sixteen patients were treated by fogarty embolectomy, 4 were treated by AngioJet aspiration, and 26 patients with mechanical thrombectomy (Rotarex system). Data from medical records of 26 patients with AEO, who typically experienced coldness of the foot, a weakened femoral artery pulse, and intermittent claudication following a walk of 300 m as confirmed by physical examination, were included in retrospective analysis. Ultrasound scans or emergency computed tomography angiography (CTA) scans showed the occlusion of left or right graft limb (Figure 1).
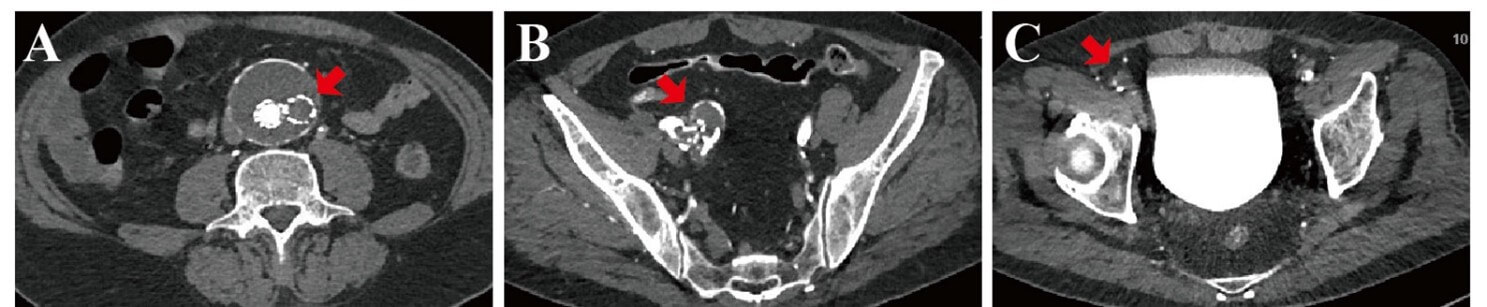
A) Occlusion and thrombosis of the right limb of the aortic endovascular stent graft (red arrow), and there was no endoleak. B) There was a twist or kink in the graft limb on the right side (red arrow). C) Occlusion and thrombosis of the right femoral artery (red arrow).
The study was approved by the ethics committee of the local medical institution and waive the informed consent. Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research. As soon as AEO was diagnosed, anticoagulation treatment was initiated and maintained during the endovascular treatment. Also, patient information, such as demographics, comorbid medical conditions, treatments, and outcomes, was recorded and analyzed.
2.2. Endovascular Treatment
Once local anesthesia was administered, either a 6F or 8F sheath was inserted via left brachial artery access or a femoral artery. In addition, an intra-arterial 3000 U and 30 U/min drip infusion of heparin were administered. We utilized both angled and straight 0.035-inch stiff wire (Terumo Corp., Leuven, Belgium) and 4F guiding catheter to pass through the occluded iliac limbs. Next, 6F or 8F 90cm sheath (Cook Inc., Bjaeverskov, Denmark) was employed to the proximal of the abdominal aortic stent, and digital subtraction angiography (DSA) was performed using a pigtail catheter to demonstrate an occluded graft limb and the distal runoff (Figure 2). At this stage, the 135cm 6F or 8F Rotarex system device (Straub Medical AG, Switzerland) was introduced, and a to-or-for movement was carefully followed at roughly 1 to 2 mm per second to aspirate all the thrombotic materials. Repeated arteriography indicated a widely patent limb with runoff to the occluded vessels.
Following the thrombectomy, balloon angioplasty was used to correct the folds, kinks, and twists. Besides, self-expanding stents or covered stents were implanted to overlap the kinking straightened stent. Completion of the DSA angiography revealed successful blood runoff.
2.3. Anticoagulation and Antiplatelet Therapy
Following the surgery, the patients had palpable femoral pulses bilaterally. Upon discharge, they were prescribed an oral 15mg anticoagulant rivaroxaban for a minimum of 6 months. On the other hand, patients with stents were prescribed clopidogrel (75 mg/d, 6 months) and were also instructed to follow a lifetime aspirin (100mg/d) therapy. Each patient underwent a general medical examination that included the ankle-brachial index (ABI) measurements, and the CTA examination (Figure 3) at 6 and 12 months.
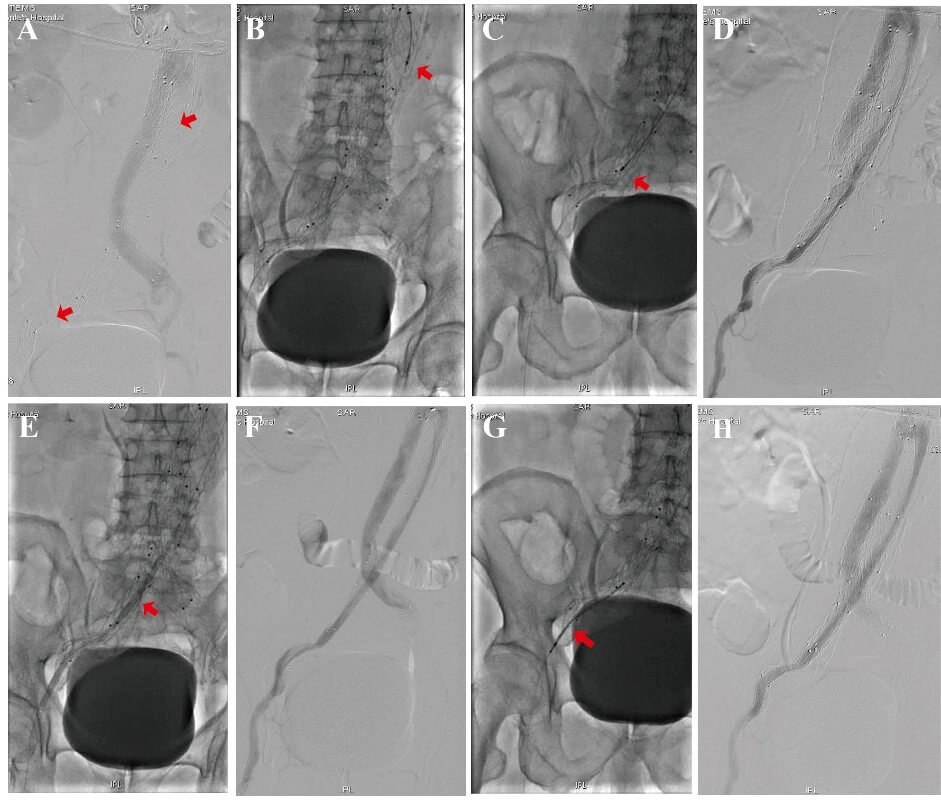
A) DSA showed complete occlusion of the right limb endograft just below the renal arteries (red arrow). B) Rotarex® thrombectomy was performed on the right limb occlusion (red arrow). C) Rotarex® thrombectomy was introduced and carefully moved through the twist and kink in the graft limb (red arrow). D) Arteriography demonstrated recanalization of the right iliac limb with some residual irregularities. E) Balloon dilatation of the twist and kink in the graft limb (red arrow). F) Arteriography demonstrated recanalization of the right iliac limb with some residual irregularities. G) A self-expanding stent was placed in the right limb twist and kinked to complete the repair (red arrow). H) Angiography showed the patency of the right graft limb.
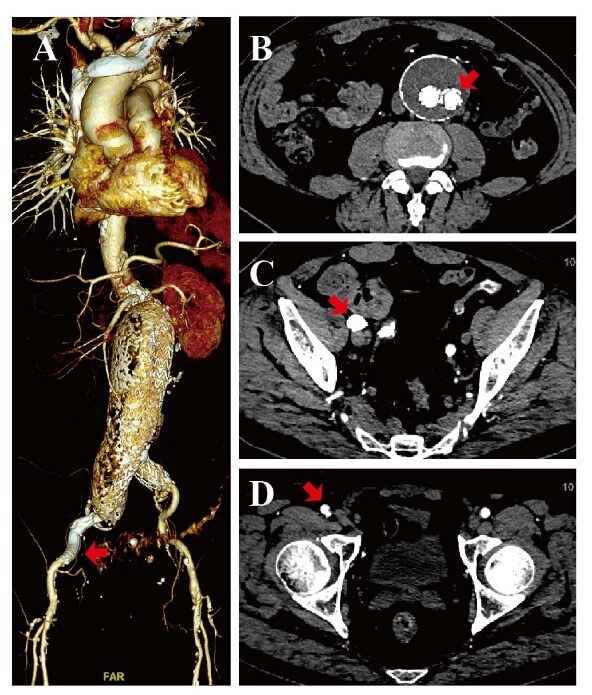
A) A 3D CTA showed the patency of the bilateral common iliac artery and the right femoral artery after 12 months (red arrow). B) Cross-section CTA showed the patency of the right limb of the aortic stent graft, and there was no occlusion and thrombosis (red arrow). C) Cross-section CTA showed the patency of the right graft limb, and the twist or kink had been remedied (red arrow). D) Cross-section CTA showed the patency of the right femoral artery, and there were no occlusions and thrombosis (red arrow).
3. Results
3.1. Patient Characteristics
There were 26 patients with aortic graft occlusion, 20 males (76.9%), and with the mean age of 67 ± 12 years (51-87). Comorbid medical conditions included hypertension that was found in 15 (57.7%) patients, diabetes mellitus in 12 (46.2%) patients, dyslipidemia in 20 (76.9%) patients, coronary artery disease in 10 (38.5%) patients, smoking in 17 (65.4%) patients, renal insufficiency in 2 (7.7%) patients, and peripheral artery disease (PAD) in 10 (38.5%) patients (Table 1). CTA indicated the occluded and extrinsic compression in the right graft limb (Figure 1).
TABLE 1: Patients’
demographics.
|
Variables |
Mean
(range) or percentage (N=26) |
|
Age
(years) |
67 ± 12
(51-87) |
|
Male |
20
(76.9%) |
|
Comorbidity |
|
|
Hypertension |
15
(61.1%) |
|
Diabetes
mellitus |
12
(46.2%) |
|
Dyslipidemia |
20
(76.9%) |
|
Coronary
artery disease |
10
(38.5%) |
|
Smoking |
17
(65.4%) |
|
Renal insufficiency |
2
(7.7%) |
|
Peripheral
artery disease |
10
(38.5%) |
Kinking or extrinsic compression was identified as the primary causes of AEO and thrombosis. Circulation was successfully restored in all patients with a 100% success rate using mechanical thrombectomy. The time taken to complete the procedure was 87 ± 25 min. Additional balloon angioplasty was performed in all the 26 patients. In 24 patients (92.3%), stenting was performed using a variety of self-expanding stents or aortic endograft placed in the graft limbs, thus re-establishing artery patency. Catheter-directed thrombolysis (250,000 U urokinase) was used to treat the residual thrombus in 2 patients (7.7%). Five patients experienced complications: 3 had peripheral embolization blue toe syndrome and 2 ischemia-reperfusion injuries. A blood loss was estimated to be approximately 300 ± 40 mL, however, blood transfusions were not needed. The period of hospitalization was 6 (4 - 13) days. Operative details and complications were listed in (Table 2).
TABLE 2: Operative
details and follow-up.
|
Operative
details |
Mean
(range) or percentage (N=26) |
|
Primary
success |
26
(100%) |
|
Balloon
angioplasty |
24
(92.3%) |
|
Stent
deployment |
24
(92.3%) |
|
Operative
time, min |
87 ± 25 |
|
Catheter-directed
thrombolysis |
2
(7.7%) |
|
Complications |
|
|
Death |
0 (0%) |
|
Blue
toe syndrome |
3
(11.5%) |
|
Ischemia
reperfusion |
2
(7.7%) |
|
Blood
loss, ml |
300 ±
40 |
|
Length
of stay, d |
6 (4 -
13) |
|
30-day
survival rate |
26
(100%) |
|
Mean
follow-up (month) |
12 (2 -
16) |
3.2. Follow-up
As mentioned before, an oral anticoagulant rivaroxaban minimally lasting for 6 months was prescribed upon discharge. Patients with stents were given additional instructions to take clopidogrel (75 mg/d, 6 months) and adhere to lifetime aspirin (100mg/d) therapy. The post-surgery CTA data obtained 12 months later showed the abdominal aorta and bilateral iliac artery patency.
4. Discussion
AEO following EVAR of AAA is a relatively common complication [1-3]. This study confirmed AEO in 46 out of the 683 patients (6.7%), which was slightly lower rate compared with the statistics in similar reports and might be explained by the highly beneficial technological advancements in the market. When considering the factors leading to AEO, Carroccio et al. have suggested that this condition is related to device kinking, migration, or device elongation to the external iliac artery [16].
Traditional treatment methods, i.e., surgical thrombectomy, has proven to be successful in the treatment of AEO [4]. However, these procedures increase the likelihood of complications, such as the risk of graft dislodgment or component separation during the operation. Femoro-femoral bypass grafting can restore perfusion to the affected limb. Yet, its longevity may be inferior to re-establishing patency of the endograft itself and is associated with the risk of failure in the endograft procedure [5]. Alternatively, catheter-directed thrombosis is related to hemorrhage. Moreover, the procedure is time-consuming and requires intensive care and repeated angiograms [17]. The AngioJet system is a rheolytic thrombectomy device that has been proven successful in eliminating thrombus from dialysis grafts, bypass grafts, and native arteries [8]. The thrombolysis is commonly used in practice for occluded grafts and arterial occlusions. However, complications, such as peripheral embolization, dissections, and bleeding, have been reported to occur in approximately 43% of cases.
The Rotarex mechanical devices are designed to disrupt and extract freshly formed thrombus from the arterial circulation [11, 18]. The Rotarex system is a purely mechanical endovascular thrombectomy device, which has a rotation capacity of up to 40000-60000 rpm. At such speed, a powerful vortex that can debulk all detachable occlusion materials created in the artery. Percutaneous mechanical thrombectomy is beneficial because it can swiftly reperfuse the ischemic limb, debulk the thrombus load, and decrease the total dosage while shortening the duration of adjunctive thrombolysis [19]. These devices have completely eliminated the need for thrombolysis in certain patients [20]. The device is highly beneficial, particularly when thrombolysis is contraindicated. Furthermore, these thrombectomy catheters are versatile and can be delivered from several access sites, including the brachial and femoral arteries. The brachial approach was commonly used in the patients involved in this study. This finding was determined based on the analysis of several clinical, noninvasive, and angiographic findings. The brachial approach considerably eases recanalization if the limb thrombosis is encountered. Lastly, this approach avoids repeated access procedures at femoral access sites.
In most of the patients, thromboses could be attributed to graft limb faults, such as extrinsic compression, focal kinking, limb twists, and/or redundant graft material [21, 22]. Kinking, which should be corrected during the initial operation, is manipulated and dilated with balloon angioplasty during the subsequent operation [1, 23]. If either kinking or a tortured iliac artery is noted, self-expanding stents or covered stents must be installed to prevent future occlusions. Additionally, a complete angiogram with the least amount of contrast medium is necessary to guarantee the success of the entire procedure.
5. Conclusion
Rotarex thrombectomy and angioplasty (balloon dilatation and stenting) are highly safe and effective in revascularizing iliac limb occlusion of aortic endograft and have a low rate of complications.
Author Contributions
Conception and design: X. Lu, J. Qin, and W. Li. Analysis and interpretation: X. Yang, L. Yuan, K. Ye, and M. Yin. Writing the article: X. Yang, L. Yuan, and X. Wu. Critical revision of the article: X. Lu, J. Qin, W. Li and M. Yin. Statistical analysis: X. Yang and J. Qin. Final approval of the version to be published: X. Yang, L. Yuan, X. Wu, K. Ye, M. Yin, X. Lu, J. Qin, and W. Li.
Acknowledgments
The authors thank Meiqiong Qian and Yu Fan, engineers from DSA Therapeutics, for their help with the intervention procedures.
Conflicts of Interest
None.
Funding
This work was supported by Natural Science Foundation of China (81971758, 81971712, 81870346), the Natural Science Foundation of Shanghai Science and Technology Committee (Grant No. 134119a2100 and 20124Y132), Shanghai Science and Technology Innovation Action Plan (20Y11909600), Clinical Research Plan of SHDC (SHDC2020CR6016-003),Shanghai Municipal Health Bureau Project (202040434),Clinical Research Program of 9th People's Hospital (JYLJ202010), and Shanghai Ninth People’s Hospital Nursing Fund Project (JYHL2020MS01).
Abbreviations
AEO: Aortic Endograft Occlusion
AAA: Abdominal Aortic Aneurysm
EVAR: Endovascular Aneurysm Repair
CTA: Computed Tomography Angiography
DSA: Digital Subtraction Angiography
REFERENCES
[1] Andreia Coelho, Clara Nogueira,
Miguel Lobo, et al. “Impact of Post-EVAR Graft Limb Kinking in EVAR Limb
Occlusion: Aetiology, Early Diagnosis, and Management.” Eur J Vasc Endovasc
Surg, vol. 58, no. 5, pp. 681-689, 2019. View at: Publisher Site | PubMed
[2] Guoquan Wang, Shuiting Zhai, Tianxiao
Li, et al. “Limb graft occlusion following endovascular aortic repair:
Incidence, causes, treatment and prevention in a study cohort.” Exp Ther Med,
vol. 14, no. 2, pp. 1763-1768, 2017. View at: Publisher Site | PubMed
[3] G K Mantas, C N Antonopoulos, G S
Sfyroeras, et al. “Factors Predisposing to Endograft Limb Occlusion after
Endovascular Aortic Repair.” Eur J Vasc Endovasc Surg, vol. 49, no. 1,
pp. 39-44, 2015. View at: Publisher
Site | PubMed
[4] Salvatore Ronsivalle, Francesca
Faresin, Francesca Franz, et al. “A new management for limb graft occlusion
after endovascular aneurysm repair adding a vollmar ring stripper: the
unclogging technique.” Ann Vasc Surg, vol. 27, no. 8, pp. 1216-1222,
2013. View at: Publisher
Site | PubMed
[5] Evan C Lipsitz, Takao Ohki, Frank J
Veith, et al. “Patency rates of femorofemoral bypasses associated with
endovascular aneurysm repair surpass those performed for occlusive disease.” J
Endovasc Ther, vol. 10, no. 6, pp. 1061-1065, 2003. View at: Publisher Site | PubMed
[6] Zvonimir Krajcer, Jerry H Gilbert,
Kathy Dougherty, et al. “Successful treatment of aortic endograft thrombosis
with rheolytic thrombectomy.” J Endovasc Ther, vol. 9, no. 6, pp.
756-764, 2002. View at: Publisher
Site | PubMed
[7] Soo Buem Cho, Ho Cheol Choi, Sang Min
Lee, et al. “Combined treatment (image-guided thrombectomy and endovascular
therapy with open femoral access) for acute lower limb ischemia: Clinical
efficacy and outcomes.” PLoS One, vol. 14, no. 11, pp. e0225136, 2019.
View at: Publisher
Site | PubMed
[8] Daniel A Leung, Lawrence R Blitz,
Teresa Nelson, et al. “Rheolytic Pharmacomechanical Thrombectomy for the
Management of Acute Limb Ischemia: Results From the PEARL Registry.” J
Endovasc Ther, vol. 22, no. 4, pp. 546-557, 2015. View at: Publisher Site | PubMed
[9] Rafael de Athayde Soares, Marcelo
Fernando Matielo, Francisco Cardoso Brochado Neto, et al. “Analysis of the Safety
and Efficacy of the Endovascular Treatment for Acute Limb Ischemia with
Percutaneous Pharmacomechanical Thrombectomy Compared with Catheter-Directed
Thrombolysis.” Ann Vasc Surg, vol. 66, pp. 470-478, 2020. View at: Publisher Site | PubMed
[10] Mariya Kronlage, Ilka Printz, Britta
Vogel, et al. “A comparative study on endovascular treatment of (sub)acute
critical limb ischemia: mechanical thrombectomy vs thrombolysis.” Drug Des
Devel Ther, vol. 11, pp. 1233-1241, 2017. View at: Publisher Site | PubMed
[11] Jinbo Liu, Tianrun Li, Wei Huang, et
al. “Percutaneous mechanical thrombectomy using Rotarex catheter in peripheral
artery occlusion diseases - Experience from a single center.” Vascular,
vol. 27, no. 2, pp. 199-203, 2019. View at: Publisher Site | PubMed
[12] Tod M Hanover, Corey A Kalbaugh,
Bruce H Gray, et al. “Safety and efficacy of reteplase for the treatment of acute
arterial occlusion: complexity of underlying lesion predicts outcome.” Ann
Vasc Surg, vol. 19, no. 6, pp. 817-822, 2005. View at: Publisher Site | PubMed
[13] Samuel Heller, Jean-Claude Lubanda,
Petr Varejka, et al. “Percutaneous Mechanical Thrombectomy Using Rotarex(R) S
Device in Acute Limb Ischemia in Infrainguinal Occlusions.” Biomed Res Int,
vol. 2017, pp. 2362769, 2017. View at: Publisher Site | PubMed
[14] Dierk Vorwerk, Stefan Triebe, Steffen
Ziegler, et al. “Percutaneous Mechanical Thromboembolectomy in Acute Lower Limb
Ischemia.” Cardiovasc Intervent Radiol, vol. 42, no. 2, pp. 178-185,
2019. View at: Publisher
Site | PubMed
[15] Paweł Latacz, Marian Simka, Paweł
Brzegowy, et al. “Mechanical rotational thrombectomy with Rotarex system
augmented with drug-eluting balloon angioplasty versus stenting for the
treatment of acute thrombotic and critical limb ischaemia in the
femoropopliteal segment.” Wideochir Inne Tech Maloinwazyjne, vol. 14,
no. 2, pp. 311-319, 2019. View at: Publisher Site | PubMed
[16] Alfio Carroccio, Peter L Faries,
Nicholas J Morrissey, et al. “Predicting iliac limb occlusions after bifurcated
aortic stent grafting: anatomic and device-related causes.” J Vasc Surg,
vol. 36, no. 4, pp. 679-684, 2002. View at: PubMed
[17] Sagar S Gandhi, Joseph A Ewing, Emily
Cooper, et al. “Comparison of Low-Dose Catheter-Directed Thrombolysis with and
without Pharmacomechanical Thrombectomy for Acute Lower Extremity Ischemia.” Ann
Vasc Surg, vol. 46, pp. 178-186, 2018. View at: Publisher Site | PubMed
[18] Krzysztof Dys, Justyna
Drelichowska-Durawa, Bartosz Dołega-Kozierowski, et al. “Mechanical
thrombectomy using Rotarex system and stent-in-stent placement for treatment of
distal femoral artery occlusion secondary to stent fracture - a case report and
literature review.” Pol J Radiol, vol. 78, no. 3, pp. 74-79, 2013. View
at: Publisher Site | PubMed
[19] Frantisek Stanek, Radoslava
Ouhrabkova, David Prochazka “Could mechanical thrombectomy replace thrombolysis
in the treatment of acute and subacute limb ischemia?” Minerva Cardioangiol,
vol. 67, no. 3, pp. 234-245, 2019. View at: Publisher Site | PubMed
[20] Michael K W Lichtenberg “Evolving
Evidence for Acute and Subacute Limb Ischemia Treatment With a Purely
Mechanical Thrombectomy Approach.” J Endovasc Ther, vol. 26, no. 3, pp.
302-304, 2019. View at: Publisher
Site | PubMed
[21] F Cochennec, J P Becquemin, P
Desgranges, et al. “Limb graft occlusion following EVAR: clinical pattern,
outcomes and predictive factors of occurrence.” Eur J Vasc Endovasc Surg,
vol. 34, no. 1, pp. 59-65, 2007. View at: Publisher Site | PubMed
[22] Jonathan D Woody, Michel S Makaroun “Endovascular graft limb occlusion.” Semin Vasc Surg, vol. 17, no. 4, pp. 262-267, 2004. View at: Publisher Site | PubMed
[23] Geert Maleux, Marcel Koolen, Sam Heye, et al. “Limb occlusion after endovascular repair of abdominal aortic aneurysms with supported endografts.” J Vasc Interv Radiol, vol. 19, no. 10, pp. 1409-1412, 2008. View at: Publisher Site | PubMed
