Received: Thu 10, Aug 2023
Accepted: Wed 30, Aug 2023
Abstract
The anterolateral thigh (ALT) flap is currently the main choice as a free flap for head and neck reconstruction, including intraoral and facial defects and it is gaining popularity in abdominal, orthopedic and pelvis reconstruction [1]. It can also be used as a pedicled flap in abdominal or perineum reconstruction. One of the most important property of the ALT flap is the low donor site morbidity, both functionally and aesthetically, compared to other workhorse flaps, thanks to the possibility to reach a direct suture in 80% of the cases. Many possibilities are currently available to cover the defect when direct suture is not achievable. A split thickness skin graft has traditionally been used to address the remaining skin defect. Due to the alteration of the thigh skin sensitivity and poor aesthetic outcomes, loco-regional flaps are gaining tremendous momentum in the field. In fact, they allow surgeons to get the donor site closure in one stage surgery, with a good tissue quality, with a like-with-like tissue. A systematic review according to the PRISMA guidelines was performed concerning the issue of donor site closure after ALT harvesting.
1. Introduction
The anterolateral thigh (ALT) flap is currently the main choice as a free flap for head and neck reconstruction, including intraoral and facial defects and it is gaining popularity in abdominal, orthopedic and pelvis reconstruction [1]. It can also be used as a pedicled flap in abdominal or perineum reconstruction [2].
This flap presents many advantages: it has a long and anatomically constant pedicle, it is pliable and allows flexible contouring, it can be harvested at different thicknesses [3]. Although the anterolateral thigh flap was originally developed as a free flap, the position of the pedicle and its wide caliber [4] allow the raising of this flap as a proximally pedicled flap for perineal for abdominal reconstruction or as a distally based pedicled flap with reverse flow for knee abnormalities or to optimize amputation stumps [5].
In addition, the anterolateral thigh flap can be raised both as a compound myocutaneous flap with simple en bloc elevation of the skin and the underlying muscle or as a muscle-sparing perforator flap by dissecting only the skin paddle [6]. Multiple tissues can be harvested on individual perforators and dissected separately but based on the same source vessel [7]; alternatively, a double-paddled flap may be raised [8] or multiple anterolateral thigh flaps harvested from a single thigh [9].
One of the most important property of the ALT flap is the low donor site morbidity, both functionally and aesthetically, compared to other workhorse flaps, thanks to the possibility to reach a direct suture in 80% of the cases [10, 11].
Different studies describe the dimension of the ALT flap that allows the donor site direct closure (i.e., flap width <7-8 cm, flap width <16% of the thigh circumference [12]), but due to the excessive skin tension and the possible outbreak of a compartmental syndrome or muscle necrosis this chance is avoided in 20% [13]. Many possibilities are currently available to cover the defect when direct suture is not achievable.
A split thickness skin graft has traditionally been used to address the remaining skin defect [14, 15] of ALT flap donor site. Skin graft is site on one of the lowest steps of the reconstructive ladder, it is easy and fast to harvest and well-known. Nonetheless, Kimata et al. [16] suggested the absence of the reducing of the range of motion (ROM) of both hip and knee joints; the alteration of the thigh skin sensitivity and poor aesthetic outcomes are likely to be complications [17]. Furthermore, there is the tendency to get a depressed scar [3].
For all these reasons, reconstructive alternatives have been described over the years, including i) tissue expanders [18], and ii) local or free flaps [19].
i) Tissue expansion could be an interesting way to reach a direct closure, but it requires time to expand the skin and sometimes, due to patient disease, that time is not available [20].
Alternatively, ii) free flaps can be used but they are difficult to implement. Loco-regional flaps are gaining tremendous momentum in the field. In fact, they allow surgeons to get the donor site closure in one stage surgery, with a good tissue quality, with a like-with-like tissue and with an easier surgical procedure compared to closing the ALT flap donor site by free flaps [17].
The interest in the abovementioned advantages and the publication of different surgical techniques (Figure 1) led us to conduct a systematic review about the loco-regional flaps reconstruction of the ALT flap donor site closure proposal, to depict the state of art of these procedures, to compare them and analyze their outcomes and feasibility. To our knowledge, this is the first systematic review about this topic.
2. Materials and Methods
A systematic review according to the PRISMA guidelines was performed. We made a literature search via PubMed using the following terms: “ALT flap donor site closure”, “ALT flap donor site closure with local flap”, ALT flap donor site direct closure”. Two Authors independently conducted the literature screening and data extraction.
2.1. Inclusion Criteria
Studies describing ALT flap donor site closure with local perforator flap, studies describing ALT flap donor site closure with random flap and studies in english.
2.2. Exclusion Criteria
Studies describing ALT flap donor site closure with free flap, studies describing ALT flap donor site closure with skin graft, studies describing ALT flap donor site direct primary closure (even if achieved with local tissue dissection), and studies describing ALT flap donor site closure by two stages surgical techniques.
2.3. Extracted Data
Number of patients, follow up (months), ALT thickness harvesting, radiological pre-operation exams, flap design, source vessel of the perforator flap, hospitalization time, number and kind of complications, defect dimension, defect width, and functional outcome.
3. Results
A total 43 manuscripts were found; hence two different authors analyzed all the 43 abstracts to identify eligible papers. The inclusion/exclusion criteria were then applied, and 10 works were selected and included in this review. The articles were all published between 2000 and 2022. All the studies selected were retrospective case-series about authors’ surgical technique proposal.
The number of patients of these series goes from 2 to 30. We considered a total of 105 ALT flap (Table 1). The main indications of the ALT flap were head and neck reconstructions (85 patients), then post-traumatic lower limb reconstructions (9 patients), post-oncological lower limb reconstructions (5 patients), and, finally, chronic wounds or necrotizing fasciitis (4 patient) or scalp defects (2 patients).
In five manuscripts the ALT flap harvesting technique is reported [17, 21-23] and only in two of these cases, both by the same author, it is supra-fascial [17, 21]. Three studies [17, 24, 25] report the supra-fascial dissection of the local flap used to close the ALT donor site, three studies describe the sub-fascial dissection [22, 26, 27], whereas in the others the technique was not specified.
Two studies [17, 25] identify the perforators by the doppler-ultrasound (color-doppler in only one [17]) and the indocyanine green (ICG) angiography. In one work a pencil-doppler is used [22], in one is specified the use of no radiological pre-operation examination [28] and in the other studies no radiological examination is mentioned.
Only 3 studies report the average time of the surgery [17, 24, 25]; in detail, just one specifies the average operation time of donor site closure harvesting the local flap [25], one includes the ALT flap harvesting [24] and the last one considers all the surgery time [28].
Only one Author describes the surgical technique of a random flap [23]; in all the other cases a perforator flap is proposed [17, 22, 24-26, 28, 29]. The majority of the authors prefer a V-Y advancement design flap [21, 22, 24, 28, 29], one describes the bipedicle flap [23] and the remaining ones use a propeller flaps (Table 2) [17, 25-28].
Only one study specifies the hospitalization time (24.5 days [28]) and the follow up is mentioned in only 4 series [17, 22, 26, 27]. The overall complication rate is 10.4% and a great variability between the studies was identified ranging from no complication [17, 25, 26] to a maximum of 6 complication in a 21 patients’ series [28]. We consider “major complication” when a surgical revision of the flap is required, whereas every complication solved by local wound care is considered as “minor complication”. All the Authors report only minor complication about their experience. In particular, only one case of infection [28] and only one case of partial flap necrosis are described [24], whereas all the other complications are represented by wound dehiscence [22, 23, 28]. No extended flap necrosis was found. No one flap required a surgical revision (Table 3).
TABLE 1: Studies
features, flap dimension, size and design, clinical evaluation and patient
follow up.
|
|
N PZ |
ALT DISSECTION |
RADIOLOGY |
AVERAGE TIME (MIN) |
RANDOM/ PERFORATOR |
FLAP DESIGN |
DONOR SITE CLOUSURE FLAP DISSECTION |
HOSPEDALIZATION
TIME (DAYS) |
DEFECT AREA (CM) |
DEFECT WIDTH (CM) |
FUNCTIONAL
OUTCOME |
AVERAGE FU
TIME (MONTHS) |
|
Yamada et al. [24]
|
10 |
- |
- |
120 (including ALT
flap elevation) |
Perforator |
V-Y advancement |
Supra-fascial |
- |
22x8 |
- |
- |
- |
|
Salgarello et
al. |
2 |
Suprafascial |
- |
- |
Perforator |
V-Y advancement |
Supra-fascial |
- |
9x12 |
9 |
No
impairment |
- |
|
Jeng et al.
|
13 |
- |
- |
- |
Perforator |
V-Y advancement |
- |
- |
50x225 |
8 |
- |
- |
|
Koshima et
al.
|
7 |
- |
Doppler
ultrasound + ICG angiography |
53min |
Perforator |
Propeller |
Supra-fascial |
- |
15x17 |
11 to 16 |
- |
- |
|
Wang et al.
|
7 |
- |
- |
- |
Perforator |
Propeller / Groin Flap |
Sub-fascial |
- |
15x5 to 17x6cm |
>7 |
No impairment |
7 |
|
Lee et
al. |
21 |
- |
None |
350.9min (including demolition time) |
Perforator |
V-Y advancement / Propeller |
- |
24.5 |
136cm2 |
7.9 |
- |
- |
|
Ellis et al. [22] |
6 |
Subfascial |
Pencil doppler |
- |
Perforator |
Key-Stone |
Sub-fascial |
- |
9.5x17.8 |
7.8 |
No impairment |
16.75 |
|
Salgarello et
al. [21] |
30 |
Suprafascial |
Color
Doppler sonography, ICG- angiography |
- |
Perforator |
Propeller |
Supra-fascial |
- |
9.5x11.5 |
9.5 |
No
impairment |
12 |
|
Kovach et al. [23] |
6 |
Subfascial
|
- |
- |
Random |
Bipedicle Flap |
- |
- |
13x10 |
8 |
- |
- |
|
Andrew T.
Huang |
3 |
Subfascial |
- |
|
Perforator |
Propeller |
Subfascial |
|
11.3x14 |
11.3 |
No
impairment |
24 |
“N”: number; ‘’-”: the
article does not specify the item; ALT: antero lateral thigh; ICG: indocyanine
green.
TABLE 2: Preferred
perforator source vessels
|
|
Ko Hosokawa,
2000 |
Marzia
Salgarello, 2013 |
Seng Feng
Jeng, 2014 |
Isao Koshima,
2017 |
Dali Wang,
2018
|
Yao-Chou Lee,
2018 |
Marco F.
Ellis, 2018 |
Marzia Salgarello,
2020 |
Andrew T.
Huang, 2020 |
|
Branches Of
LCFA |
10 |
|
13 |
- |
4 |
13 |
- |
5 |
1 |
|
SFA/PFA Perforator |
- |
|
- |
- |
- |
- |
6 |
- |
2 |
|
AMT Perforator |
- |
2 |
- |
- |
- |
8 |
- |
10 |
|
|
TFL Perfortor |
- |
|
- |
- |
- |
- |
- |
15 |
|
|
SCIA |
- |
|
- |
- |
3 |
- |
- |
- |
|
TABLE 3: Complication
rate.
|
|
Wound Dehiscence |
Partial Flap Necrosis |
Infection |
Extended Flap Necrosis
|
|
Ko Hosokawa, 2000 |
- |
1 |
- |
- |
|
Salgarello,
2013 |
- |
- |
- |
- |
|
Seng Feng Jeng, 2014 |
- |
- |
1 |
- |
|
Isao Koshima,
2017 |
- |
- |
- |
- |
|
Dali Wang, 2018 |
- |
- |
- |
- |
|
Yao-Chou Lee,
2018 |
6 |
- |
- |
- |
|
Marco F. Ellis, 2018 |
1 |
- |
- |
- |
|
Marzia
Salgarello, 2020 |
- |
- |
- |
- |
|
Stephen J. Kovach, 2020 |
1 |
- |
- |
- |
|
Andrew T.
Huang |
1 |
- |
- |
- |
Finally, five authors underline the absence of lower limb functional impairment closing the ALT donor site by loco-regional flaps as long term complication, in the other manuscripts this is not highlighted [17, 21, 22, 26, 27].
4. Discussion
Thanks to its unique characteristics, ALT flap is an actual workhorse in reconstructive surgery. The primary donor site closure is considered the best surgical option to close the donor site, but it is difficult to achieve when the defect exceeds 8 cm in width or 16% of thigh circumference [12]. The high-tension closure is related to increasing rate of compartment syndrome and muscle necrosis [13]. Although the repair with skin grafts is an easy, quick and widely used solution; the scar contractures, increased rate of hip and knee range of motion (ROM) and poor aesthetic outcomes are inevitable consequences [17].
Hosokawa et al. first introduced the ALT flap donor site defect closure by a loco-regional flap describing their technique in a 10 patients series. They harvested the flap basing its vascularization on the profunda artery or on the descending branch of lateral circumflex artery [24]. Jeng et al. [29] achieved a V-Y antegrade or retrograde advancement flap to close the donor site when direct closure is not available. To the best of our knowledge, this is the first time an author describes the use of the transverse branch of the lateral circumflex femoral artery (LCFA) to cover the central portion of the ALT flap donor site.
Despite the use of retrograde flap having the highest risk of venous congestion due to poor flow through the venous valves, no venous congestion has been reported [30, 31]. In this field, the use of propeller flaps occurred only in 2017, described first by Koshima et al. [25]. This technique was then adopted by Wang et al. [26], Salgarello et al. [17], Lee et al. [28]. As clarified by the authors, the use of the propeller flaps allows a maximal mobility of the flap up to 180° rotation, which facilitates the closure of an ALT defect with minimal flap size. Additionally, they decrease dogear [25] deformity and do not add scars to the thigh [17].
Propeller flaps are a solution increasingly mentioned in literature; in fact the possibility to have more stable and aesthetically better donor site covers, unchanged surgical times and no additional scars represents an undoubted advantage [32].
Lee et al. describe the possibility to close the ALT flap donor site with the anteromedial thigh (AMT) V-Y advancement flap or an AMT propeller flap. They use the AMT flaps whenever there are no residual vessels in the antero-lateral tight surface. In particular, a propeller flap is harvest when a great flap mobilization is necessary to close the defect. Pagliara et al. [17] similarly propose a propeller flap from the medial thigh with a 90° rotation whenever a medial thigh perforator close to the defect is found. It should be underlined that the AMT perforator flap, both harvest as advancement and as propeller, involves the presence of an additional scar on the medial thigh [17].
Wang et al. describe the use of groin flap to close the defect. Although this technique seems tempting and the possibility to set up large skin paddle with minimal visible scars is possible, the author himself clarifies how the groin flap used as a local flap is limited by the lack of a long vascular pedicle. Consequently, repairing thigh skin defects by using a groin flap is limited to defects position on the upper-to-mid third of the thigh [19]. Random flaps, such as the keystone flap [21, 22] or the bipedicle flap [23], are constant, safe, and easily to set up; however, they left multiple and longer scars.
Koshima et al. [25] propose the supercharge propeller flap by anastomosing the ligated vessels to the nearby small muscle ones [25]. This procedure reduces the risk of venous congestion, but excellent microsurgical skills are required. Regarding the pre-operation radiological exams, three works describe the use of a doppler to localize the perforators; moreover, two of these add the verification of the perforosome vascularization by the ICG. In our experience, when harvesting an ALT flap both an angio-TC and a handle-doppler examination are performed, but we do not verify the perforosome vascularization by the ICG. Even though both the works with the ICG angiography use report no donor site complications, there are not enough cases to establish if there is a real advantage in using this exam. Despite the confirmation of flaps vascularization pattern by using ICG being well described in literature [33, 34], in our opinion, in this field, the dimension of the flap to harvest and the cost of the procedure do not allow to introduce it as an every-day resource. It is to note that the complication rate reported in the other works is low, indeed.
The dissection plane was described in five articles: three authors prefer the supra-fascial dissection, two authors perform the sub-fascial one, whilst in the other works it is not declared. Wound healing and infections represent the most common complications. Other complications like hematoma and seroma are not recorded. Hung et al. [28] reported 8.8% of complications, all represented by wound dehiscence. When complications occur, ALT donor site is usually treated with local debridement, dressing, and eventually with skin graft [28].
The necrosis of rectus femoris muscle and compartment syndrome are dramatic although rare complications by closing the ALT donor site defect by direct suture [10, 28]. Harvesting a local flap allows exploiting the excess of the thigh skin tissue sharing the traction forces on the thig and reducing the risk of the compartment syndrome outbreak. No cases of compartment syndrome or rectus femoris muscle have been reported in the studies we analyzed, indeed.
Our review shows how the V-Y advancement perforator flap is the preferred design, and the branches of the lateral circumflex femoral artery are the most chosen source vessels. This kind of flap has several advantages, including the constant presence of a perforator close to the defect and the possibility to cover wide surface without exert excessive traction on the pedicle, thanks the laxity of the medial thigh tissue.
Unfortunately, we found only few information about the specific surgical time to harvest and inset these loco-regional flaps, about the hospitalization time of patient or patient mobilization time (Table 1). In fact, none of the published studies declare if the proposed technique improves or lengthens the overall surgical time. Figure 1 summarizes the various local flaps through which an ALT donor-site may be closed.
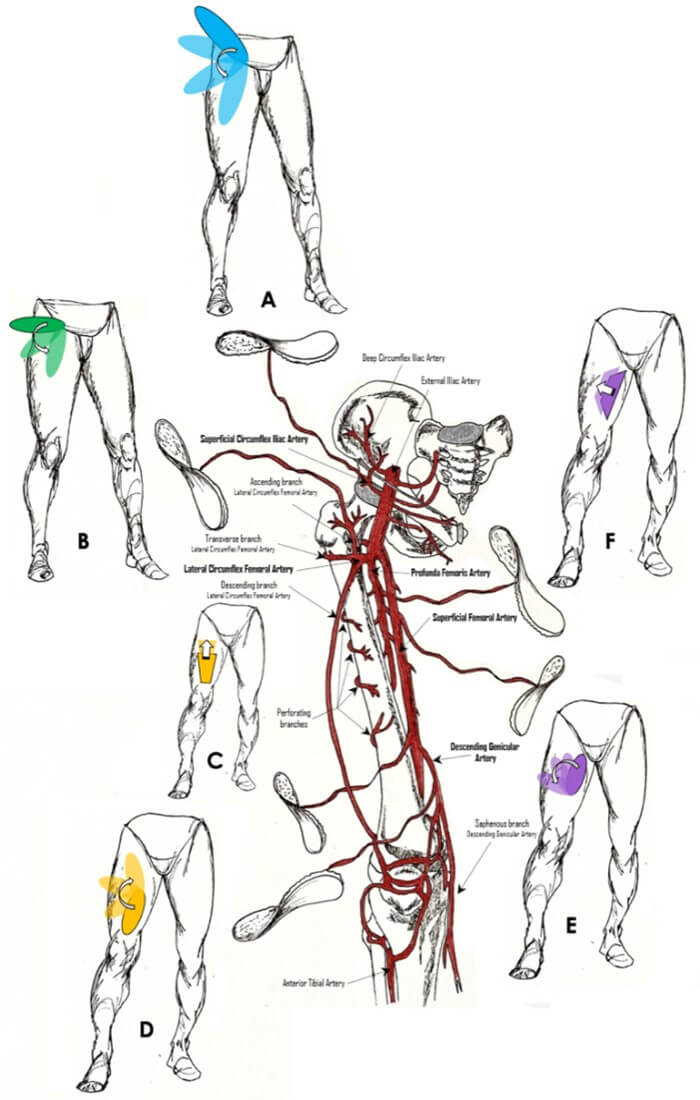
It would be interesting to compare the surgical and the hospitalization time of the various donor site closure techniques to highlight the best choice in these terms. Finally, in all the studies examined, the number of patients is relatively small. The use of local flaps to close the ALT donor site is not the treatment of choice for most surgeons. Good anatomical knowledge and microsurgery training are essential elements.
5. Conclusion
ALT flap is one of the most used flaps in various reconstructive surgery fields. There are many possibilities to close this flap donor site when direct suture is not available, such as skin grafting, tissue pre-expansion, local or free flaps. This review, the first in literature, collects the different loco-regional pedicled flaps used to close the ALT flap donor site. According to our review, the loco-regional flaps should be likely considered to closure the ALT flap donor site when a primary closure is not achievable, bringing into the defect high quality and like-with-like tissues, reaching good outcomes and without suffering a high rate of complication. Future studies should deepen in the average surgery time and comparing these flaps each other to establish the best one.
Conflicts of Interest
None.
Funding
None.
Abbreviations
ALT: Antero Lateral Thigh
ROM: Range of Motion
ICG: Indocyanine Green
LCFA: Lateral Circumflex Femoral Artery
AMT: Anteromedial Thigh
REFERENCES
[1] Fu-chan Wei, Vivek Jain, Naci Celik,
et al. “Have we found an ideal soft-tissue flap? An experience with 672
anterolateral thigh flaps.” Plast Reconstr Surg, vol. 109, no. 7, pp.
2219-2226, 2002. View at: Publisher Site | PubMed
[2] Andreas I Gravvanis, Dimosthenis A
Tsoutsos, Dimitrios Karakitsos, et al. “Application of the pedicled
anterolateral thigh flap to defects from the pelvis to the knee: Pedicled
Anterolateral Thigh Flap.” Microsurgery, vol. 26, no. 6, pp. 432-438,
2006. View at: Publisher
Site | PubMed
[3] Joon Pio Hong, Dong Hoon Choi,
Hyunsuk Suh, et al. “A New Plane of Elevation: The Superficial Fascial Plane
for Perforator Flap Elevation.” J. Reconstr. Microsurg, vol. 30, no. 7,
pp. 491-496, 2014. View at: Publisher
Site | PubMed
[4] Rozina S Ali, Rachel
Bluebond-Langner, Eduardo D Rodriguez, et al. “The Versatility of the
Anterolateral Thigh Flap.” Plast Reconstr Surg, vil. 124, no. 6 Suppl,
pp. e395-e407, 2009. View at: Publisher Site | PubMed
[5] Gang Zhou, Qi-Xu Zhang, Guang-Yu Chen
“The earlier clinic experience of the reverse-flow anterolateral thigh island
flap.” Br J. Plast Surg, vol. 58, no. 2, pp. 160-164, 2005. View at: Publisher Site | PubMed
[6] Fu-Chan Wei, Naci Celik, Seng-Feng
Jeng “Application of ‘simplified nomenclature for compound flaps’ to the
anterolateral thigh flap.” Plast Reconstr Surg, vol. 115, no. 4, pp.
1051-1055, 2005. View at: Publisher
Site | PubMed
[7] Yu-Te Lin, Chih-Hung Lin, Fu-Chan Wei
“More degrees of freedom by using chimeric concept in the applications of
anterolateral thigh flap.” J Plast Reconstr Aesthet Surg, vol. 59, no.
6, pp. 622-627. View at: Publisher
Site | PubMed
[8] Wei-Chao Huang, Hung-Chi Chen, Vivek
Jain, et al. “Reconstruction of through-and-through cheek defects involving the
oral commissure, using chimeric flaps from the thigh lateral femoral circumflex
system.” Plast Reconstr Surg, vol. 109, no. 2, pp. 433-441, 2002. View
at: Publisher
Site | PubMed
[9] Erh-Kang Chou, Betul Ulusal, Ali
Ulusal, et al. “Using the descending branch of the lateral femoral circumflex
vessel as a source of two independent flaps.” Plast Reconstr Surg, vol.
117, no. 6, pp. 2059-2063, 2006. View at: Publisher
Site | PubMed
[10] Tommaso Agostini, Davide Lazzeri,
Giuseppe Spinelli “Anterolateral thigh flap: Systematic literature review of
specific donor-site complications and their management.” J. Craniomaxillofac
Surg, vol. 41, no. 1, pp. 15-21, 2013. View at: Publisher Site | PubMed
[11] Georgia-Alexandra Ch Spyropoulou,
Pao-Yuan Lin, Chih-Yen Chien, et al. “Reconstruction of the Hypopharynx with
the Anterolateral Thigh Flap: Defect Classification, Method, Tips, and Outcomes.”
Plast Reconstr Surg, vol. 127, no. 1, pp. 161-172, 2011. View at: Publisher Site | PubMed
[12] Radovan Boca, Yur-Ren Kuo, Ching-Hua
Hsieh, et al. “A Reliable Parameter for Primary Closure of the Free
Anterolateral Thigh Flap Donor Site.” Plast Reconstr Surg, vol. 126, no.
5, pp. 1558-1562, 2010. View at: Publisher Site | PubMed
[13] Patrick D Addison, Declan Lannon,
Peter C Neligan “Compartment Syndrome After Closure of the Anterolateral Thigh
Flap Donor Site: A Report of Two Cases.” Ann Plast Surg, vol. 60, no. 6,
pp. 635-638, 2008. View at: Publisher Site | PubMed
[14] Matthew M Hanasono, Roman J Skoracki,
Peirong Yu “A prospective study of donor-site morbidity after anterolateral
thigh fasciocutaneous and myocutaneous free flap harvest in 220 patients.” Plast
Reconstr Surg, vol. 125, no. 1, pp. 209-214, 2010. View at: Publisher Site | PubMed
[15] Joan E Lipa, Christine B Novak, Paul
A Binhammer “Patient-reported donor-site morbidity following anterolateral
thigh free flaps.” J Reconstr Microsurg, vol. 21, no. 6, pp. 365-370,
2005. View at: Publisher
Site | PubMed
[16] Y Kimata, K Uchiyama, S Ebihara, et
al. “Anterolateral Thigh Flap Donor-Site Complications and Morbidity.” Plast
Reconst. Surg, vol. 106, no. 3, pp.584-589, 2000. View at: Publisher Site | PubMed
[17] Domenico Pagliara, Maria Lucia
Mangialardi, Stefano Vitagliano, et al. “Improving Outcomes in Anterolateral
Thigh Flap Donor-Site Reconstruction Using Propeller Flaps: A Retrospective
Comparative Study with Skin Grafting.” J Reconstr Microsurg, vol. 37,
no. 5, pp. 436-444, 2020. View at: Publisher Site | PubMed
[18] Andrew G Silver, Richard C Baynosa
“Utilization of a continuous external tissue expansion system to assist in
primary closure of a large anterolateral thigh donor site defect.” Case Rep
Surg, vol. 2014, pp. 860749, 2014. View at: Publisher Site | PubMed
[19] Dedi Tong, Yuanbo Liu, Lehao W Wu, et
al. “Free groin flap for aesthetic and functional donor-site closure of the
anterolateral thigh flap.” J Plast Reconst. Aesthet Surg, vol. 69, no.
8, pp. 1116-1120, 2016. View at: Publisher Site | PubMed
[20] Geoffrey G Hallock “The Preexpanded
Anterolateral Thigh Free Flap.” Ann Plast Surg, vol. 53, no. 2, pp.
170-173, 2004. View at: Publisher
Site | PubMed
[21] Giuseppe Visconti, Marzia Salgarello
“Anteromedial thigh perforator-assisted closure of the anterolateral thigh free
flap donor site.” J Plast Reconstr Aesthet Surg, vol. 66, no. 7, pp.
e189-e192, 2013. View at: Publisher
Site | PubMed
[22] Sergey Y Turin, Jamie A Spitz, Karina
Alexander, et al. “Decreasing ALT donor site morbidity with the keystone flap.”
Microsurgery, vol. 38, no. 6, pp. 621-626, 2018. View at: Publisher Site | PubMed
[23] Merisa L Piper, John T Stranix, John
H Bast, et al. “A Bipedicled Flap for Closure of the Anterolateral Thigh Flap
Donor Site.” Plast Reconstr Surg Glob Open, vol. 8, no. 8, pp. e2770,
2020. View at: Publisher
Site | PubMed
[24] N Yamada, M Kakibuchi, H Kitayoshi,
et al. “A new way of elevating the anterolateral thigh flap.” Plast Reconstr
Surg, vol. 108, no. 6, pp. 1677-1682, 2001. View at: Publisher Site | PubMed
[25] Takuya Iida, Hidehiko Yoshimatsu,
Isao Koshima “Reconstruction of Anterolateral Thigh Defects Using
Perforator-Based Propeller Flaps”. Ann Plast Surg, vol. 79, no. 4, pp.
385-389, 2017. View at: Publisher
Site | PubMed
[26] Chengliang Deng, Hai Li, Zairong Wei,
et al. “Various surgical techniques to create an aesthetic appearance at the
donor site of anterolateral thigh free flaps based on the oblique branch:
Twenty-one clinical case reports.” Medicine (Baltimore), vol. 97, no. 7,
pp. e9885, 2018. View at: Publisher
Site | PubMed
[27] Caroline S Hudson, Andrew T Huang
“Perforator‐based propeller flaps for
reconstruction of massive anterolateral thigh donor site wounds.” Head Neck,
vol. 42, no. 12, pp. E49-E52, 2020. View at: Publisher Site | PubMed
[28] Kuo-Shu Hung, Szu-Han Chen, Wei-Chen
Chen, et al. “Surgical Algorithmic Approach to Facilitate Primary Closure of
the Anterolateral Thigh Flap Donor Site in Head and Neck Reconstruction.” Ann
Plast Surg, vol. 82, no. 1S Suppl 1, pp. S33-S38, 2019. View at: Publisher Site | PubMed
[29] Jaime Eduardo Pachón Suárez, Parviz
Lionel Sadigh, Hsiang-Shun Shih “Achieving Direct Closure of the Anterolateral
Thigh Flap Donor Site-An Algorithmic Approach.” Plast Reconstr Surg Glob
Open, vol. 2, no. 10, pp. e232, 2014. View at: Publisher Site | PubMed
[30] Il-Kug Kim, Tae-Gon Kim, Jun-Ho Lee,
et al. “The Clinical Course of Reverse-flow Anterolateral Thigh Flap: Need to
Prepare for Venous Congestion and Salvage Operation.” Arch Plast Surg,
vol. 39, no. 3, pp. 262-264. View at: Publisher Site | PubMed
[31] Chan-Su Kang, Tae-Gon Kim
“Distally-based free anterolateral thigh flap with a modified vena comitans.” Arch
Plast Surg, vol. 46, no. 1, pp. 84-87, 2019. View at: Publisher Site | PubMed
[32] Mario F Scaglioni, Alberto Franchi,
Elmar Fritsche “Propeller flap donor site closure by means of another propeller
flap: A case report and literature review.” Microsurgery, vol. 40, no.
2, pp. 252-257, 2020. View at: Publisher
Site | PubMed
[33] Pierre Burnier, Jérémy Niddam, Romain Bosc “Indocyanine green applications in plastic surgery: A review of the literature.” J Plast Reconstr Aesthet Surg, vol. 70, no. 6, pp. 814-827, 2017. View at: Publisher Site | PubMed
[34] Ke Li, Zheng Zhang, Fabio Nicoli, et al. “Application of Indocyanine Green in Flap Surgery: A Systematic Review.” J Reconstr Microsurg, vol. 34, no. 2, pp. 77-86, 2018. View at: Publisher Site | PubMed
